Table 3.
Cleavage of the redox auxiliary.a
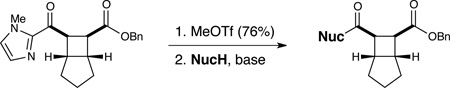 | |||
|---|---|---|---|
| entry | NucH | yieldb | dr |
| 1c | H2O | 52%c | >10:1 |
| 2c | MeOH | 86% c | >10:1 |
| 3 | i-PrOH | 88% | >10:1 |
| 4 | t-BuOH | 0% | n.d. |
| 5 | t-BuSH | 99% | >10:1 |
| 6d | BnNH2 | 98% | >10:1 |
| 7d | pyrrolidine | 75% | >10:1 |
Unless otherwise noted, cleavage of the imidazolium group was conducted using an excess of the nucleophile and 3.5 equiv of DBU in CH2Cl2.
Isolated yields.
Cleavage conducted in Et2O.
No DBU added.
