Figure 1. A model of the housekeeping Sec and accessory SecA2 export systems.
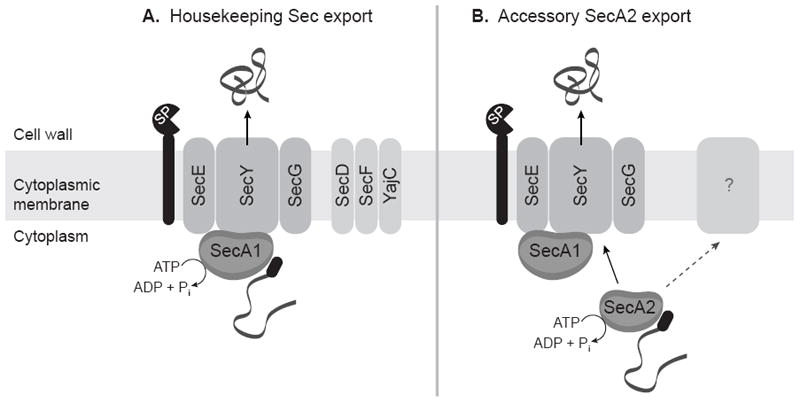
(A) Preproteins with N-terminal signal peptides (black oval) are recognized by SecA1, which interacts with the SecYEG channel complex to form the translocase. SecA1 performs repeated cycles of ATP hydrolysis, pushing the unfolded preprotein through the SecYEG channel. SecD, SecF, and YajC increase efficiency of protein export. Signal peptides are removed by a LepB or LspA signal peptidase (SP), and mature proteins fold into their final conformations. (B) SecA2 recognizes a small subset of proteins and uses its ATPase activity to assist in their export. In the most likely scenario, SecA2 works with components of the housekeeping Sec export system and exports proteins across the cytoplasmic membrane through the SecYEG channel complex. However, it remains possible that other unknown components are required in addition to or in lieu of the housekeeping Sec components. The role of SecA2 in the export of proteins lacking signal peptides (not shown) is currently not understood.
