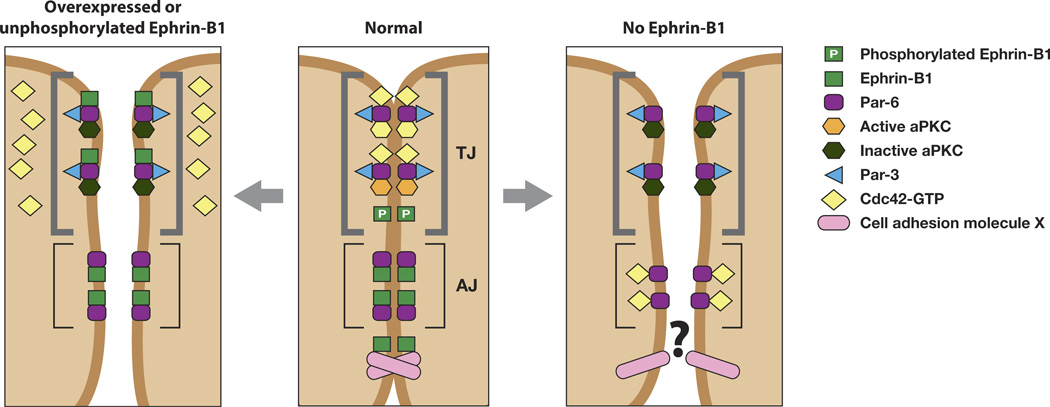
Figure 3.
Ephrin-B1 regulates tight junction formation through an interaction with Par-6. Unphosphorylated ephrin-B1 may compete with activated Cdc42 for binding to Par-6. Lack of a Par-6/Cdc42 interaction inhibits aPKC activation, leading to tight junction disruption (left panel). Tyrosine phosphorylation of ephrin-B1 precludes an interaction with Par-6, leaving it available to interact with Cdc42-GTP and establish tight junctions (middle panel). In the absence of ephrin-B1, Par-6 that is localized to adherens junctions and lateral cell borders may compete with tight junction localized Par-6 for active Cdc42. This may effectively reduce available Cdc42 at the apical border, resulting in a reduction of aPKC activity and disrupt tight junctions (right panel). Alternatively, an as yet unknown cell adhesion molecule that normally associates with ephrin-B1 may no longer function in the absence of ephrin-B1, resulting in loss of TJs and AJs. (Figure adapted from [24]).