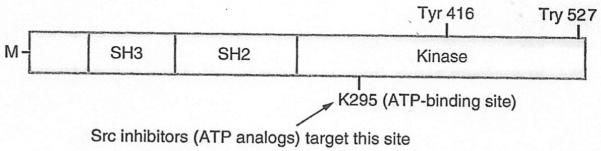
Figure 3. Src tyrosine kinase structural domains.
The Src protein has three major structural domains that mediate its functions: the SH2 domain to which molecules with tyrosine residues bind; the SH3 domain, which binds proline-rich portions of interacting molecules, and the kinase domain, which phosphorylates tyrosines on target proteins. Autophosphorylation of tyrosine 416 keeps the Src kinase in an active conformation while phosphorylation of tyrosine 527 has the opposite effect. Tyrosine 295 (K295) is in the ATP-binding site and is essential for Src kinase activity. The N-terminal region of Src is M, which allows it to be associated with the cell membrane.
M: Myristoylated; SH: Src homology.