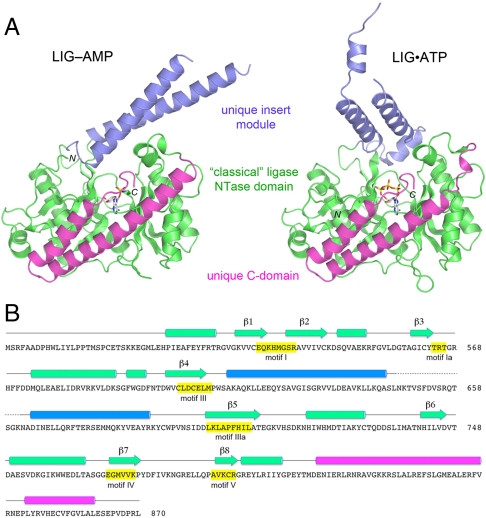
Fig. 4.
Tertiary structure and component domains of CthLIG. (A) CthLIG is composed of three domains: a classical ligase nucleotidyl transferase domain (green), a unique insert domain (blue) within the NTase, and a unique C-terminal domain (magenta). The LIG-AMP and LIG•ATP (protomer A) structures were superimposed and then offset horizontally. N and C specify the amino and carboxyl termini of the LIG polypeptides. The nucleotide in the active site is shown as a stick model. (B) The secondary structure elements of LIG-AMP [colored by domain as in (A)] are aligned above the amino acid sequence of CthLIG, with β strands rendered as arrows and α helices as cylinders. The nucleotidyltransferase motifs are highlighted in yellow boxes.