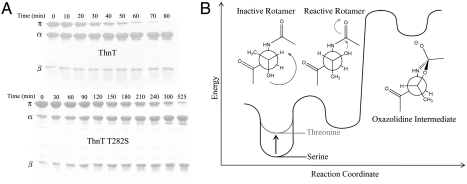
Fig. 2.
The reactive rotamer effect in autoproteolysis. (A) Time course of the autoproteolysis reactions of ThnT and T282S shown on 15% SDS-PAGE. As the reaction progresses, there is a decrease in the intensity of the uncleaved (π) band and a corresponding increase in the intensity of the two cleavage products (α and β). Quantification with first-order kinetics shows a 4.3-fold rate deceleration caused by removal of the γ-methyl. (B) Hypothetical reaction coordinate diagram showing how threonine accelerates nucleophilic attack into the N-terminal amide bond by minimizing the number of gauche interactions for the reactive rotamer. This effect is specific to the 3R stereochemistry of the β-carbon and is also influenced by the geometry of the local protein backbone.