NK cells of the pregnant mouse uterus acquire CD127 at mid-gestation and may become responsive to lymphoid organ stromal factors produced by decidua.
Keywords: decidua, interleukin 7, stromal factor, thymic stromal lymphopoietin
Abstract
Decidualization, a progesterone-dependent process that alters endometrial stromal cells at implantation sites in humans and rodents, is accompanied by a highly regulated, NK cell-dominated leukocyte influx into decidual basalis (DB). Whether uNK cells differentiate from uterine progenitor cells is unknown, as are the mechanisms restricting leukocytes to DB. We asked if cells expressing the early NK lineage marker CD127 (IL-7Rα) occurred in mouse decidua. CD127 was absent from gd6.5 decidual lymphoid cells but became expressed by a mature uNK cell subset in gd10.5 DB. DB and transient myometrial structures (MLAp) that ring maternal blood vessels supplying placentae expressed IL-7 and TSLP, the CD127 ligands, but with differing temporal and spatial patterns. UNK cells expressed TSLPR, and study of gd10.5 implantation sites from mice deleted for IL-7, CD127, or TSLPR suggested that IL-7 and its receptor have physiological roles in limiting expansion of immature uNK cells within MLAp, while the TSLP signaling pathway is used in DB to sustain IFN-γ production from a subset of mature uNK cells. Regionalized, dynamic expression of the additional lymphoid organ stromal markers gp38/podoplanin and ER-TR7, but not CD157, were seen by immunohistochemistry in implantation sites, and DB and MLAp contained transcripts for Aire, a tolerance-promoting factor. These observations suggest that CD127+ NK lineage progenitors are not present in the early postimplantation period of mouse uterus and that decidualized endometrial stroma has key immunoregulatory properties.
Introduction
Uterine (u)NK cells are abundant, transient lymphocytes found in the decidualizing endometrium of early pregnancy in species with hemochorial placentation, such as human, rat, and mouse [1, 2]. Relationships between uNK and other NK cells are incompletely defined. It remains unclear whether the large uNK cell population arises from circulating or uterine progenitor/precursor cells. In mice, transplantable uNK progenitor cells have been identified in primary and secondary lymphoid tissues, including thymus and LN but not in uterine segments [1, 3]. UNK cells play key roles in the endometrial remodeling associated with pregnancy, including roles in promotion of maternal angiogenesis and transformation of the major maternal arterial branches supplying the placenta, called spiral arteries [2, 4]. UNK cells are thought to interact with and share these functions with conceptus-derived placental trophoblast cells [4] and to have paracrine effects that alter gene expression in uterine stromal fibroblasts [5]. In contrast to human uNK cells that appear late in each menstrual cycle and are identified as CD56bright [6], mouse uNK cells appear after blastocyst implantation (gd4) [1] and are identified as DBA lectin-reactive [7].
Flow cytometry has resolved human and mouse NK cells into subsets that reflect progressive lineage differentiation and maturation [8, 9]. Application of this paradigm to human uNK cells suggested that only later, nonmultipotent NK cell stages were present in cycling endometrium and in first trimester decidua [10]. CD34−CD117+CD94− NK cells were the earliest stage identified in human uterus, a phenotype similar to “Stage 3” NK cells in secondary lymphoid organs. Many CD34−CD117+CD94− human uterine cells strongly expressed CD127 (IL-7Rα) [10], a marker proposed as a thymic emigrant tag [11, 12]. CD127 is also expressed by mouse NK lineage cells early during differentiation [9]. When CD27 and CD11b are used to describe the progression of mouse NK cell differentiation, the earliest doubly negative cells are 50% CD127+. At the next stage of differentiation (CD11blow CD27high), characterized transcriptionally as highly proliferative, 32% of immature NK cells is CD127+. At later stages of NK cell maturation, when the transcriptome is biased toward effector functions, CD127 expression is rare (1–2%). Thus, if mouse uNK precursors reside in the uterus, some of them should be identifiable as CD127+, particularly during their proliferative expansion that occurs initially within early gd5.5–8.5 DB and later within the MLAp [7].
Recently, unique CD127+ NK progenitor cells were indentified in mouse LN that differentiate by thymic-dependent and thymic-independent pathways [13]. These murine CD127+ NK cells, like human CD56highCD16− NK cells, produce significant amounts of cytokines but only become cytotoxic after long-term activation [14, 15]. As human LN is a known precursor source for CD56bright NK cells [16], and mouse uNK cells differentiate from LN [17], we asked whether CD127 expression was present on uNK cells in mouse implantation sites. UNK cells were not CD127-reactive at gd6.5 but in a time course study, were shown to acquire CD127 with terminal differentiation. The CD127 ligands IL-7 and TSLP (thymic stromal lymphopoietin) were locally expressed by decidual cells, and genetic blockade of these signaling pathways differentially altered uNK cells and their synthesis of IFN-γ.
MATERIALS AND METHODS
Mice
B6, BALB/c, CD-1 (Jackson Laboratory, Bar Harbor, ME, USA), and locally bred alymphoid Rag2−/−/Il2rg−/− mice on a BALB/c background were housed under barrier conditions at Queen's University (Canada). For timed matings, estrous females were selected, paired to syngeneic males, and checked for copulation plugs the following morning. The morning of plug detection was named gd0.5. Gd10.5 uteri from syngeneic matings of B6 background Tslpr−/− (NIH, Bethesda, MD, USA), Il7−/− (University of Toronto, Canada), and Il7rα−/− (CD127 null; University of British Columbia, Canada) were shipped to Queen's University. All procedures were conducted under Animal Utilization Protocols approved by the appropriate institutional Animal Care Committees.
Histological procedures
Females mated at Queen's University were deeply anesthetized and then perfused with 30 ml freshly prepared 4% PFA in buffered saline. Pregnant uteri were dissected, transected between implantation sites, and postfixed in PFA (15 min for gd6.5–10.5 sites, 60 min for gd12.5 sites). Standard procedures were used for automated processing into paraffin, embedding and cutting of 7 μm serial sections. Two or more viable implantation sites from each of multiple [2–5] females were studied for each gd reported. Fetal liver was collected as a positive control tissue and processed similarly.
Blood vessel morphometry and uNK cell enumeration were conducted as described previously [1, 18, 19] using 11 slides/implantation site and three implantation sites/pregnancy. Slides for vascular measurements were stained with H&E; slides for uNK cell enumeration were stained with biotinylated DBA lectin (1 μg/ml; Sigma-Aldrich, St. Louis, MO, USA), followed by streptavidin peroxidase and DAB to identify the granules and plasma membranes of uNK cells. Specificity control incubations incorporated N-acetylgalactosamine, the sugar that blocks DBA lectin binding. For some slides, FITC- or TRITC-conjugated DBA lectin (1 μg/ml; Sigma-Aldrich) was used with epifluorescence for uNK cell visualization. Additional slides were stained with Periodic Acid Schiff's reagent to recognize uNK. cell glycoproteins. Mathematic arterial wall thickness was calculated from: thickness = [square root (vessel area/π) − square root (lumen area/π)].
Immunohistochemistry for CD127 and TSLP was undertaken using preplacental gd6.5, 7.5, or 8.5 implantation sites or placental gd10.5 or 12.5 implantation sites. After deparaffinization and rehydration, peroxidases were inactivated (3% H2O2 in 0.1 M PBS, 6 min), and a blocking reagent was applied (1% BSA in PBS, 20 min) and then replaced by a primary rabbit antibody against CD127 (Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:200) or TSLP (Sigma-Aldrich; 1:400) overnight (4°C). After rinsing, slides were incubated with biotinylated goat anti-rabbit IgG (Dako, Denmark; 30 min) and then visualized with DAB (Sigma-Aldrich) or with Alexa Fluor 488/594 goat anti-rabbit IgG (Invitrogen, Carlsbad, CA, USA; 1:200) when dual staining with FITC- or TRITC-conjugated DBA lectin was being conducted (Sigma-Aldrich; 1:200). Coverslips were mounted using Permount (Fisher Scientific, Waltham, MA, USA) for immunohistochemistry or ProLong Gold anti-fade reagent (Invitrogen) for immunofluorescence. Negative controls were serial sections processed identically, except the primary antibody was replaced by nonimmune rabbit serum. For stromal cell study, gd7.5 and 10.5 implantation sites were stained with anti-mouse CD157, ER-TR7 (thymic reticulum, Erasmus University Rotterdam, The Netherlands; BMA Biomedicals, Switzerland), or hamster anti-mouse gp38 (RELIATech, Germany). All images were collected and analyzed using an AxioImager M.1 microscope and Axiovision software (Carl Zeiss, Oberkochen, Germany).
BM transplantation
Three, 6-week-old BALB/c-Rag2−/−/Il2rg−/− females were pretreated with 150 mg/kg 5-fluorouracil, i.p., 48 h before i.v. infusion of 2 × 107 BALB/c leukocytes flushed from BM. Three to 4 weeks later, these recipients were mated for study at gd6.5 (n=2) or 12.5 (n=3).
Flow cytometry
Viable gd10.5 BALB/c implantation sites were dissected to isolate DB and MLAp. Each tissue was then mechanically dissociated to provide cell suspensions for flow cytometry, as described elsewhere [20]. After incubation (30 min) in normal rat serum, leukocytes were stained (30 min, 4°C) with FITC-conjugated DBA (1 μg/ml; Sigma-Aldrich) and antibodies purchased from eBioscience (San Diego, CA, USA): PE-conjugated anti-mouse CD122 (5H4; 1 μg/ml), PE-Cy5-conjugated anti-mouse CD3 (145-2C11; 1 μg/ml), and APC-Alexa Fluor-conjugated anti-mouse CD127 (A7R34; 1.2 μg/ml) or tagged isotype control IgG. Fifty thousand events were collected using Cytomics FC500 flow cytometry (Beckman Coulter, Brea, CA, USA), and data were processed with FlowJo software (Tree Star, Ashland, OR, USA). Cell suspensions were prepared from mechanically dissociated gd10.5 BALB/c female spleen, thymus, and BM and labeled similarly.
cDNA synthesis and PCR protocols
To evaluate Tslp and Tslpr expression in uNK cells, FACS-sorted CD3−CD122+ DBA+ uNK cells were studied. In brief, decidual lymphocytes of gd10.5 CD-1 mice were isolated and stained with FITC-conjugated DBA, PE-conjugated CD122, and PE-Cy5-conjugated CD3.
CD3−CD122+DBA+ uNK cells were collected by EPICS Altra Flow HyPerSort Cytometer (Beckman Coulter). Then, RNA was isolated, reverse-transcribed, and amplified using the Ovation Pico WTA System (NuGEN, San Carlos, CA, USA) to obtain cDNA, which was used as the PCR template. PCR amplification used the Qiagen PCR kit with the following conditions: 94°C for 3 min (one cycle); 94°C for 30 s, 55°C for 30 s, 72°C for 30 s (40 cycles), and 72°C for 10 min (one cycle). PCR products were separated on 1.0% agarose gel and visualized by ethidium bromide staining. RNA was also prepared from the MLAp, DB and thymus of gd10.5 B6 mice to examine expression of Aire. Primer sequences were given below. All PCR products were confirmed by sequencing.
Tslp (266 bp), 5′-CCAGGCTACCCTGAAACTGA-3′ (forward),
5′-TCTGGAGATTGCATGAAGGA-3′ (reverse);
Tslpr (267 bp), 5′-CCGGCTTCCCGTTTTCGGCT-3′ (forward),
5′-AAGTTGGCGCCGTGGTGGTC-3′ (reverse);
Aire [21], 5′-TGTGCCACGACGGAGGTGAG-3′ (forward),
5′-GGTTCTGTTGGACTCTGCCCTG-3′ (reverse).
For quantitative RT-PCR, total RNA was extracted from gd10.5 B6 DB or MLAp using the Qiagen RNeasy Mini Kit. cDNA was synthesized from 1.5 μg total RNA using Invitrogen SuperScript III First-Strand Synthesis System. Then, 20 ng cDNA was subjected to real-time PCR in 96-well plates as triplicates, according to the manufacturer's protocol [10 min at 95°C, 40 cycles of 5 s at 95°C for denaturing, and 33 s at 60°C for annealing and extension using iTaq Fast SYBR Green Supermix with ROX (Bio-Rad Laboratories, Hercules, CA, USA) and ABI Prism 7500 (Applied Biosystems, Foster City, CA, USA)]. Primer sequences were given below, and products were confirmed by sequencing:
Il7 [22], 5′-TGGAATTCCTCCACTGATCC-3′ (forward),
5′-ACCAGTGTTTGTGTGCCTTG-3′ (reverse);
Cd127 [23], 5′-TCTGACCTGAAAGTCGTTTATCGC-3′ (forward),
5′-CATCCTCCTTGATTCTTGGGTTC-3′ (reverse);
Tslp [24], 5′-CCAGGCTACCCTGAAACTGA-3′ (forward),
5′-TCTGGAGATTGCATGAAGGA-3′ (reverse);
Hprt1, 5′-GCTGACCTGCTGGATTACAT-3′ (forward),
5′-TTGGGGCTGTACTGCTTAAC-3′ (reverse).
Relative expression levels of target transcripts were normalized to Hprt1 transcripts.
Statistical analysis
Data are expressed as mean ± sd. Student's t test was applied for statistical analysis; P values of <0.05 were considered significant.
RESULTS
CD127 expression in B6 implantation sites between gd6.5 and gd12.5
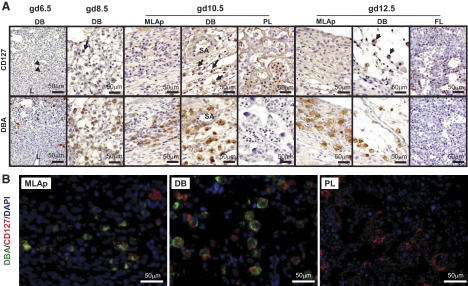
Serial sections from gd6.5 to gd12.5 B6 implantation sites were stained with CD127 or/and DBA lectin (Fig. 1A and B). At gd6.5, an occasional CD127 signal (arrowheads) was found on decidual stromal cells but not DBA+ uNK cells. At gd8.5, some DBA+ uNK cells were very weakly CD127-reactive. At gd10.5 (midgestation), CD127+ uNK cells were present. These were more frequent in DB than in MLAp. Endothelium and smooth muscle cells of the spiral arterial walls were CD127−. Gd10.5 placentas were also CD127-reactive over trophoblast cells and some nucleated fetal blood cells. By gd12.5, when uNK cell numbers are in decline, CD127 reactivity appeared to be weaker over uNK cells in DB and barely detectable on uNK cells in the MLAp. Gd12.5 fetal liver, used as a positive control tissue, contained CD127-reactive cells.
Figure 1. CD127 expression in midsagittal serial sections from gd6.5 to gd12.5 B6 implantation sites.
(A) Immunohistochemistry for CD127 (upper) or DBA-lectin (lower). (B) Immunofluorescence staining of gd10.5 B6 implantation site. Detection was nuclear DNA, DAPI (blue); uNK cells, DBA lectin (green); and CD127 antibody-reactive cells (red). DBA+ uNK cells (brown) were present in gd6.5 DB, but none of these or other cells with a lymphoid appearance expressed detectable CD127 (A). Rare stromal cells were CD127+ (arrowheads). At gd8.5, the DBA+ uNK cell population contained infrequent, weakly stained CD127+ cells (arrow). At gd10.5, when uNK cells peak, many strongly stained CD127+DBA+ uNK cells (arrows) were present in DB. At gd12.5, CD127+DBA+ uNK cells remained in DB (arrows) and were now detected in MLAp. Trophoblast cells were CD127+DBA−, as shown for gd10.5 placenta (PL). Gd12.5 fetal liver (FL) was used as a positive control for CD127. Two or more viable implantation sites from each of multiple [2–5] females were studied at each gd. L, Residual uterine lumen; SA, spiral artery.
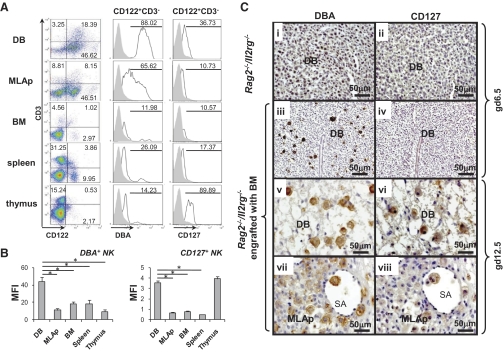
To quantify CD127 expression by DBA+ uNK cells, flow cytometry was undertaken using gd10.5 BALB/c mice (Fig. 2A). Approximately one-half of the leukocytes from DB or MLAp was CD3−CD122+ cells (putative NK cells), which were 4–15 times more than thymus, spleen, or BM. Analyses of CD3−CD122+ cells for DBA lectin expression revealed significantly more DBA+CD3−CD122+ uNK cells from DB (88%) than in uNK cells from the MLAp (66%; P<0.05). Many fewer surface DBA+CD3−CD122+ cells were present in lymphoid tissues. The parallel analysis of frequency of CD3−CD122+ cells for CD127 expression revealed the highest frequency of this subset in thymus (90%), as reported by others [15], and gd10.5 DB had the second-highest frequency (37%), followed by spleen. The MLAp and BM had similar frequencies of CD127+CD3−CD122+ cells. Analysis of MFI revealed that expression of glycosylation sites binding DBA lectin was significantly higher on CD3−CD122+ NK cells in DB than on uNK cells in the MLAp or in any other tissue (P<0.05). UNK cells from DB again showed greater MFI than uNK cells from MLAp, BM, or spleen (P<0.05). MFI for CD127 on NK cells from the thymus was similar to that for NK cells from DB (Fig. 2B). These data suggest that midpregnancy DB but not MLAp is a specialized site that briefly supports CD127+ NK cells. The CD127+ cells appear from their size—side-scatter profile indicative of granularity and positional location to be mature—activated NK cells rather than progenitors or early lineage cells.
Figure 2. CD127 expression in different mouse tissues.
(A) Isolated uterine lymphocytes were stained with FITC-conjugated DBA lectin, PE-conjugated CD122 (5H4), PE-Cy5-conjugated CD3 (145-2C11), and APC-Alexa Fluor-conjugated CD127 (A7R34). CD3−CD122+ NK cells were further gated to analyze their DBA and CD127 expression. Plots representative of three experiments are shown. Percentages of DBA+ or CD127+ populations among gated CD3−CD122+ cells are shown. (B) MFI of DBA+, CD127+ NK (CD3−CD122+) cells in various tissues. *P < 0.05. (C) Photomicrographs of implantation sites from Rag2−/−/Il2rg−/− mice without or with engraftment of normal BM, at different gd. Serial sections were stained with DBA-lectin (brown, left side) or CD127 (brown, right side). WT gd6.5 Rag2−/−/Il2rg−/− implantation sites had no DBA+ or CD127+ uNK cells (i, ii). After engraftment, DBA+ uNK cells lacking CD127 expression were present at gd6.5 (iii, iv). At gd12.5, graft-derived DBA+ uNK cells populated DB (v), and MLAp (vii). graft-derived CD127+ uNK cells were found at gd12.5 in interstitial and intravascular areas of DB (vi, viii); n = 3.
To confirm that homed uNK cell progenitors indeed lack CD127 when the lineage is established but acquire the capacity to express CD127 at midgestation, CD127 immunohistochemistry was undertaken on implantation sites from Rag2−/−/Il2rg−/− recipients of BALB/c BM. In the absence of transplantation, implantation sites in this strain lack uNK cells (Fig. 2C, i). CD127+ uNK cells were undetectable at gd6.5 in nontransplanted and in transplanted Rag2−/−/Il2rg−/− recipients, although DBA+ NK cells were numerous in the transplant recipients. CD127+ uNK cells were detectable in transplant recipients at gd12.5 (Fig. 2C). For the gd12.5 transplant-derived CD127+ uNK cells, it was again observed that their frequency and staining intensity were greater in DB than in MLAp.
CD127 ligands in mouse implantation sites
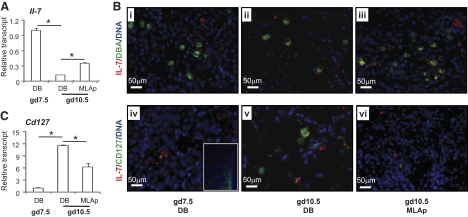
CD127 is a component of two cytokine receptors: IL-7, paired with IL-2Rγ, and TSLP, paired with TSLPR [25]. UNK cells do not differentiate in mice genetically deleted for Il15 or its receptor Il2rγ [26], and this has been attributed to the absence of IL-15 signaling [27, 28], as previous work indicated that Il7 mRNA was absent from mouse decidua between gd3.5 and gd18 [29]. Using our primers, Il7 mRNA was detected in gd7.5 B6 DB and in gd10.5 DB and MLAp (Fig. 3A). Transcripts were relatively more abundant at gd7.5 than at gd10.5, and at gd10.5, Il7 transcripts were more abundant in the MLAp than in DB. Immunohistochemistry confirmed IL-7 production at both times (Fig. 3B, i–iii). At gd10.5 but not gd7.5, a few DBA+ uNK cells were among the IL-7+ cell population. IL-7+ uNK cells were more frequent in DB than in MLAp. CD127 transcripts were relatively more abundant at gd10.5 than at gd7.5, and at gd10.5, transcript abundance was greater in the DB than in the MLAp (Fig. 3C). Dual immunohistochemistry for IL-7 and CD127 at gd7.5 showed nonoverlapping patterns of reactive cells with IL-7+ cells in the central DB and CD127+ cells in the lateral sinusoid regions. At gd10.5, CD127+ cells comingled with IL-7+ cells in central DB but were not detectable in MLAp (Fig. 3B, iv–vi).
Figure 3. IL-7 and CD127 in B6 implantation sites.
(A) Real-time PCR analysis of relative Il7 transcript abundance in gd7.5 DB and in gd10.5 DB and MLAp. (B, i–vi) Immunofluorescence colocalization of IL-7 (red) with DBA+ uNK cells or CD127+ cells (green). IL-7 was detected in DB. CD127 was detected in lateral DB at gd7.5 (iv; inset). Nuclear DNA was detected by DAPI (blue). (C) Real-time PCR analysis of relative abundance of Cd127 (Il7rα) transcripts at gd7.5 in DB and at gd10.5 in DB and MLAp. (A and C) Data are shown as mean ± sd. *P < 0.05.
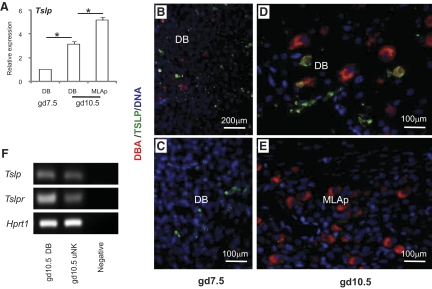
Tslp transcripts were detected in gd7.5 DB and in gd10.5 DB and MLAp (Fig. 4A). Transcript abundance was greater in both gd10.5 tissues than in gd7.5 DB and statistically higher in gd10.5 MLAp than DB. TSLP was localized in gd7.5 and gd10.5 implantation sites by immunohistochemistry. At gd7.5, TSLP+ decidual cells and DBA+ uNK cells did not appear to interact (Fig. 4B; higher power 4C; and Supplemental Fig. 1). At gd10.5, TSLP+ cells were more frequent in DB than MLAp, an outcome inverse to that predicted from RNA analysis. Gd10.5 DB was the only region where an occasional DBA+ uNK cell costained with anti-TSLP. Using mRNA extracted from FACS-isolated CD3−CD122+DBA+ uNK cells, Tslp and Tslpr expression by uNK cells was confirmed (Fig. 4F).
Figure 4. TSLP expression in B6 implantation sites.
(A) Relative Tslp expression analyzed by real-time PCR. (B and C) Immunohistochemical detection of TSLP at gd7.5 in DB at lower and higher power revealed that TSLP+ decidual cells (green) were not DBA+ uNK cells (red). At gd10.5, TSLP reactivity was present in DB and with some colocalization to DBA+ uNK cells (D) but barely detectable in MLAp (E). Nuclear DNA detected by DAPI (blue). (F) PCR detection of Tslp and Tslpr expression in homogenates of gd10.5 DB and in FACS-sorted CD3−CD122+DBA+ uNK cells. Data are shown as mean ± sd. *P < 0.05.
To address the functional significance of Il-7 and Tslp signaling pathways in implantation sites, histological and morphometric studies were conducted on gd10.5 uteri from Il7−/−, Il7rα−/−, and Tslpr−/− mice and then compared with normal B6 implantation sites. UNK cell numbers in DB were not altered from control in any gene-deleted strain (Fig. 5A). However, uNK cell numbers in the MLAp were elevated in Il7−/− and Il7rα−/− but not in Tslpr−/− implantation sites. Additionally, decidual spiral arterial modification, a feature linked with uNK cell-derived IFN-γ, was not impaired (arterial wall thickness to vessel lumen ratios are given in Supplemental Fig. 2B). This outcome was surprising, as immunohistochemistry for IFN-γ, which reaches its peak in normal implantation sites at gd10.5 and is largely uNK cell-derived, was undetectable in gd10.5 Tslpr−/− uNK cells (Fig. 5E and I; Supplemental Fig. 2A). In contrast, IFN-γ-producing DBA+ uNK cells were abundant in DB of gd10.5 implantation sites from B6, Il7−/−, and Il7rα−/− mice (Fig. 5B–D and F–H).
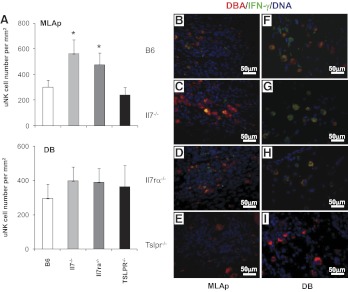
Figure 5. Distribution and IFN-γ reactivity of uNK cells in gd10.5 B6, Il7−/−, Il7rα−/−, and Tslpr−/− mice.
(A) uNK cell enumeration in MLAp and DB. More uNK cells (DBA+) were present in the MLAp of Il7−/−, Il7rα−/− mice; no differences were present in DB between the strains. (B–I) Immunofluorescent staining was used to identify DBA+ uNK cells (red) and IFN-γ (green) in the MLAp and DB. IFN-γ was undetectable in Tslpr−/− implantation sites. Nuclear DNA was detected by DAPI (blue). Two or more viable implantation sites from each of multiple [2–5] females were studied. Data are shown as mean ± sd. *P < 0.05.
Additional immune-like properties of decidual stroma
To determine whether decidua expresses molecules other than TSLP, which are associated with lymphoid organ stroma, immunohistochemistry of gd7.5 and gd10.5 B6 implantation sites was undertaken using antibodies against CD157 (a marker of B cell stroma), gp38 (Podoplanin, a marker of stroma in T cell regions), and ER-TR7 (an ECM protein produced by lymphoid tissue reticular fibroblasts). All antibodies reacted appropriately in spleen (not shown). No CD157+ decidual stroma was found (not shown), consistent with the known, profound deficit in B cells in DB [30]. For gp38 and ER-TR7, intense mesometrial reactivity was observed, although neither antibody stained cells of the central DB (Fig. 6A–D). At gd7.5, gp38 was expressed circumferentially by the myometrium and lateral DB and was not associated with uNK cells (inset). At gd10.5, gp38 reactivity remained present across the entire myometrium and was strong in the MLAp, where many uNK cells appeared to be surrounded by gp38+ stromal cells (Fig. 6B). When CD127 replaced DBA lectin as a cell marker in specimens costained with gp38, no CD127 expression was detected in the central DB at gd7.5. However, CD127+ cells were found in lateral sinusoid of the gd 7.5 implantation site and closely associated with gp38+ stromal cells (Fig. 6E and F). At gd10.5, gp38+ cells were distributed mainly in B6 MLAp, and the pattern was unaltered in Il7−/−, Il7rα−/−, or Tslpr−/− mice (Fig. 6B and G, and Supplemental Fig. 3). The pattern of ER-TR7 localization overlapped with that of gp38 at both time-points studied (Fig. 6C and D). Importantly however, ER-TR7+ cells were present in the central DB and had contact with gd10.5 DBA+ uNK cells (Fig. 6D, inset).
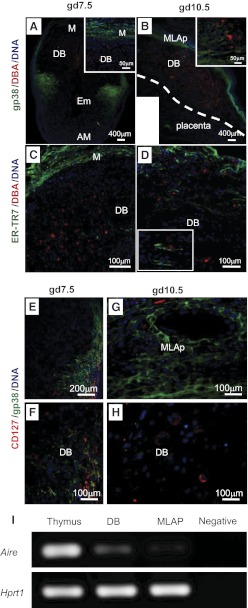
Figure 6. Stromal networks and uNK cells in gd7.5 and gd10.5 B6 implantation sites.
Immunofluorescent reactivity using DBA lectin (red) and gp38/podoplanin (green; A and B) or ER-TR7 (green; C and D) identified decidual stromal regions expressing lymphoid stroma-associated markers and their relationships with uNK cells. The relationships between CD127+ cells and the gp38+ microenvironment are shown at gd7.5 (E and F) and at gd10.5 (G and H). DAPI (blue) was used to localize cell nuclei. (I) PCR detected transcripts of Aire in adult nonpregnant thymus, DB, and MLAp. H2O served as the negative control. M, Mesometrial side; AM, antimesometrail side; Em, embryo. Scale bars indicate image magnification. Two or more viable implantation sites from each of multiple [2–5] females were studied.
Lymphoid organ stromal cells have important roles in regulation of tolerance and immune responses [31]. We hypothesized that the distinct and dynamic stromal microdomains we observed within decidua might be components of a structural network promoting tolerance of the conceptus. We therefore asked if DB and MLAp expressed Aire, a gene associated with induction of self-tolerance. As illustrated in Fig. 6I, Aire transcripts were detected in gd10.5 B6 DB and MLAp, providing support for further exploration of this hypothesis.
DISCUSSION
The goal of this study was to determine if CD127+ NK lineage progenitor cells were present in the mouse uterus during decidual differentiation. At gd6.5, DBA+ uNK cells, including small, early, nongranulated cells with an average diameter of 9 μm [7], were present, but none were CD127+. This observation suggests that uNK cells do not differentiate directly from CD127+ progenitor cells migrating from the LN or thymus. Alternatively, this observation could indicate that resident uterine progenitors of uNK cells do not share their steps in differentiation with NK cells found in LN, thymus, marrow, spleen, liver, or blood, as the earliest stages do not express CD127 [32]. The latter interpretation seems unlikely, as genetic deletions that ablate peripheral NK cells block uNK cell differentiation. A simpler hypothesis that supports conclusions drawn in human pregnancies [10] is that only relatively mature NK cells are able to leave the circulation, enter decidual tissue, and become specialized uNK cells. Only 1–2% of later-stage CD11bhighCD27high/low NK cells are CD127+ in other mouse organs [9]. If some of these cells enter decidua, their frequency may have been below detection by immunohistochemistry.
Unexpectedly, many uNK cells became CD127+ by gd10.5, and their frequency was unevenly distributed between the maternal mesometrial subregions. There were threefold more CD127+ uNK cells in DB than in MLAp. Morphometric and ultrastructural approaches have estimated that central DB has 10% early, 70% mature, and 20% early senescent uNK cells, whereas MLAp has 90% immature, 10% mature, and no senescent uNK cells [7]. The finding that 37% of uNK cells in gd10.5 DB were CD127+ suggests that not all mature uNK cells induce or sustain CD127 expression. Yadi et al. [33], using a similar mechanical dissociation protocol and FACS analyses, found no CD127+ uNK cells in gd9.5 uteri. However, our immunohistochemistry and flow cytometric data clearly show developmentally regulated CD127 expression with the critical gain in expression between gd9.5 and gd10.5. Highly significant changes occur in conceptus development and in maternal physiology at gd9.5–10.5. At this time, fusion of the allantois to the chorion opens the placental circulation [34], maternal spiral arterial modification occurs [1], and declining maternal blood pressure reaches its nadir and begins increasing [35]. All of these features would alter perfusion and oxygenation of the uNK cell environment.
To address whether CD127 acquisition is biologically important in uNK cell function, midgestation implantation sites in mice deleted for the receptor were studied. In comparison with control B6 implantation sites, uNK cell frequency in Il7rα−/− mice was increased in MLAp but normal in DB, suggesting that one or both of the cytokines using CD127 are important in limiting recruitment, expansion, or differentiation of immature uNK cells. As Il7−/− but not Tslpr−/− mice matched in phenotype, this action is attributed to IL-7. Dual antibody immunohistochemistry was used to address expression of IFN-γ by gd10.5 DBA+ uNK cells. B6, Il7−/−, and Il7rα−/− decidua contained dually positive cells of similar appearance. Tslpr−/− decidua lacked detectable IFN-γ-producing uNK cells, despite abundant, nongranulated, and granulated uNK cells. In T cells, TSLP and IL-7 activate STAT5, the former via JAK1 and JAK2 and the latter via JAK1 and JAK3 [36]. STAT5 directly promotes Ifng transcription through binding to multiple conserved, noncoding sequences identified in a region extending 60–70 kb upstream and downstream of the mouse Ifng locus and binding to the Ifng promoter [37]. These epigenetic changes facilitate T-bet binding to the Ifng promoter. With STAT5 common to both cytokine signaling pathways, it is not obvious why uNK cell production of IFN-γ differs between the mutant strains studied. Eomes rather than T-bet appears to be the dominant transcription factor used by uNK cells to regulate Ifng gene expression [38]. Thus, the importance of specific regulatory elements in preventing IFN-γ production in the absence of Tslpr remains an open question, although RUNX3 and GATA3 are strong candidates [37, 39]. Failure to detect IFN-γ in uNK cells of Tslpr−/− mice was expected to result in failure of normal, midgestational, spiral arterial modification, as seen in Rag2−/−/Il2rg−/− females with uNK cells differentiated from Ifng−/− BM donors [40]. This was not observed; instead, fully modified spiral arteries were present. At present, there is no explanation for this observation, except unmasking of other cytokine interactions and compensation at the fetal-maternal interface. Trophoblast cells contribute to decidual vascular remodeling but anticytokeratin staining of gd10.5 Tslpr−/− implantation sites to define the position of trophoblast did not reveal overinvasion or trophoblast in the vicinity of the dilated arteries (unpublished results). Further, our IFN-γ immunohistochemistry excluded other cells as providing compensatory IFN-γ in Tslpr−/− decidua. Thus, IFN-γ and intravascular trophoblast invasion are not the only mechanisms promoting gestational spiral arterial remodeling. A possible reason for late onset of CD127 expression in some uNK cells is suggested by the above observations. That is, gain of CD127 by a subset of uNK cells is a “savior” signal that prolongs their viability as IFN-γ-producing cells at the time when widespread senescence is triggered in uNK cells [41]. This could be a mechanism to provide gradual withdrawal of IFN-γ from its peak levels in decidua.
TSLP was originally described as a thymic stromal cell line product; it is now recognized as prevalent in epithelial cells [42]. Previous reports about TSLP in mouse and human implantation sites have focused on trophoblast and DCs [43]. To ascertain whether endometrial decidualization initially creates a trophoblast-free, mesometrial domain that not only recruits specific leukocyte subsets but may also regulate their responsiveness, we sought positional information on additional lymphoid tissue stromal markers. Expression of CD157, a molecule important in B cell and granulocyte extravasation in secondary lymphoid tissues [31, 44], was not detected. This is consistent with reports that B cells and granulocytes are very rare in gd7.5 decidua [45], especially in barrier-husbandry-raised mice (unpublished results). Gp38 and ER-TR7, markers of reticular fibroblasts found in T cell zones of secondary lymphoid tissue, were detected. In gd7.5 and gd10.5 implantation sites of the normal and null strains investigated, both markers were strongly associated with the uterine wall (myometrium) but were not polarized mesometrially where leukocytes accumulate. gd7.5 central DB lacked gp38, but it was expressed strongly in transient antimesometrial decidua and in the lateral decidua, where large vascular sinuses occur. Scattered ER-TR7-reactive cells were present in gd7.5 central DB, where unique endothelium expressing only VCAM-1 is reported and considered to be important for recruitment of uNK cells and intravascular trophoblasts [46]. In contrast, TSLP was localized to the central DB rather than myometrium at gd7.5. Thus, stromal markers that contribute to compartmentalization of lymphoid organs have unique distributions in gd7.5 mouse implantation sites.
At midpregnancy (gd10.5), relationships between the stroma and uNK cells had changed. Myometrium and the newly differentiated MLAp region were strongly gp38+ and ER-TR7+, suggesting that regulation of uNK cell differentiation within the MLAp may share features with T cell differentiation within lymphoid tissues. Immature uNK cells of the MLAp were surrounded by gp38+ cells, whereas more mature uNK cells in the DB, many now CD127+, had no association. CD127+ non-uNK cells, which may have been DCs [47], continued to associate with gp38+ stroma in the lateral decidua. TSLP continued to be detected at midgestation in the central DB, where ER-TR7+ cells appeared. TSLP was more abundant at gd7.5 than gd10.5 and in DB than MLAp, the inverse of relative transcript abundance, suggesting post-transcriptional regulation of TSLP in implantation sites. Detection of Aire indicated that decidual stroma may have more similarities to thymic stroma than simply production of TSLP and perhaps participates in a number of ways to gestational tolerance of the genetically distinct conceptus [48]. Of note, LN endothelial cells have roles in Aire-independent tolerance induction [49]. Early decidua is an exceptionally vascular tissue, and its endothelial cells may also have this capability, particularly when the conclusion suggested from our study is that the mouse uNK cell lineage is established by decidual homing of relatively mature CD127+ NK cells. In sum, this study has shown that neither IL-7 nor TSLP is essential for uNK development during normal pregnancy. However, each of these cytokines has a distinct, functional impact on uNK cells in separate mesometrial regions of implantation sites.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Natural Sciences and Engineering Research Council, Canada and Canadian Institutes of Health Research (B.A.C.), the Canada and Canadian Institutes of Health Research Canada (MOP#67157; J.H.F and J.L.G), the Canada Research Chairs Program (B.A.C.), Province of Ontario/Queen's Postdoctoral Fellowship (J.Z.), and the Intramural Research Program, National Heart, Lung, and Blood Institute, National Institutes of Health (Y.R. and W.J.L). We thank Mr. K. Hatta and Drs. G. N. Smith and C. Tayade (Queen's University) for helpful discussions and Dr. C. Paige and Mr. S. Corfe (University of Toronto) for provision of pregnant Il7−/− uteri.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- Aire
- autoimmune regulator
- B6
- C57BL/6J
- BM
- bone marrow
- DB
- decidual basalis
- DBA
- Dolichos biflorus agglutinin
- gd
- gestation day
- Hprt1
- hypoxanthine guanine phosphoribosyl transferase 1
- MFI
- median fluorescence intensity
- MLAp
- mesometrial lymphoid aggregate of pregnancy
- TSLP
- thymic stromal lymphopoietin
- uNK
- uterine NK
AUTHORSHIP
J.Z. designed the study, performed experiments, analyzed data, and wrote the manuscript. Z.C. performed experiments and analyzed results. J.H.F., Y.R., A.W.P., and N.A. prepared samples and performed experiments. J.H.F., W.J.L., and J.L.G. discussed the results and edited the manuscript. B.A.C. designed the study, discussed the results, and edited the manuscript.
REFERENCES
- 1. Croy B. A., van den Heuvel M. J., Borzychowski A. M., Tayade C. (2006) Uterine natural killer cells: a specialized differentiation regulated by ovarian hormones. Immunol. Rev. 214, 161–185 [DOI] [PubMed] [Google Scholar]
- 2. Moffett-King A. (2002) Natural killer cells and pregnancy. Nat. Rev. Immunol. 2, 656–663 [DOI] [PubMed] [Google Scholar]
- 3. Chantakru S., Miller C., Roach L. E., Kuziel W. A., Maeda N., Wang W. C., Evans S. S., Croy B. A. (2002) Contributions from self-renewal and trafficking to the uterine NK cell population of early pregnancy. J. Immunol. 168, 22–28 [DOI] [PubMed] [Google Scholar]
- 4. Hanna J., Goldman-Wohl D., Hamani Y., Avraham I., Greenfield C., Natanson-Yaron S., Prus D., Cohen-Daniel L., Arnon T. I., Manaster I., Gazit R., Yutkin V., Benharroch D., Porgador A., Keshet E., Yagel S., Mandelboim O. (2006) Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 12, 1065–1074 [DOI] [PubMed] [Google Scholar]
- 5. Germeyer A., Sharkey A. M., Prasadajudio M., Sherwin R., Moffett A., Bieback K., Clausmeyer S., Masters L., Popovici R. M., Hess A. P., Strowitzki T., von Wolff M. (2009) Paracrine effects of uterine leucocytes on gene expression of human uterine stromal fibroblasts. Mol. Hum. Reprod. 15, 39–48 [DOI] [PubMed] [Google Scholar]
- 6. King A., Burrows T., Verma S., Hiby S., Loke Y. W. (1998) Human uterine lymphocytes. Hum. Reprod. Update. 4, 480–485 [DOI] [PubMed] [Google Scholar]
- 7. Paffaro V. A., Jr., Bizinotto M. C., Joazeiro P. P., Yamada A. T. (2003) Subset classification of mouse uterine natural killer cells by DBA lectin reactivity. Placenta 24, 479–488 [DOI] [PubMed] [Google Scholar]
- 8. Aharon G., Freud M. A. C. (2006) Human natural killer cell development. Immunol. Rev. 214, 56–72 [DOI] [PubMed] [Google Scholar]
- 9. Chiossone L., Chaix J., Fuseri N., Roth C., Vivier E., Walzer T. (2009) Maturation of mouse NK cells is a 4-stage developmental program. Blood 113, 5488–5496 [DOI] [PubMed] [Google Scholar]
- 10. Male V., Hughes T., McClory S., Colucci F., Caligiuri M. A., Moffett A. (2010) Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. J. Immunol. 185, 3913–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Di Santo J. P., Vosshenrich C. A. J. (2006) Bone marrow versus thymic pathways of natural killer cell development. Immunol. Rev. 214, 35–46 [DOI] [PubMed] [Google Scholar]
- 12. Di Santo J. P. (2006) Natural killler cell developmental pathways: a question of balance. Annu. Rev. Immunol. 24, 257–286 [DOI] [PubMed] [Google Scholar]
- 13. Luther C., Warner K., Takei F. (2011) Unique progenitors in mouse lymph node develop into CD127+ NK cells: thymus-dependent and thymus-independent pathways. Blood 117, 4012–4021 [DOI] [PubMed] [Google Scholar]
- 14. Shi F-D., Ljunggren H-G., La Cava A., Van Kaer L. (2011) Organ-specific features of natural killer cells. Nat. Rev. Immunol. 11, 658–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vosshenrich C. A. J., Garcia-Ojeda M. E., Samson-Villeger S. I., Pasqualetto V., Enault L., Goff O. R-L., Corcuff E., Guy-Grand D., Rocha B., Cumano A., Rogge L., Ezine S., Di Santo J. P. (2006) A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat. Immunol. 7, 1217–1224 [DOI] [PubMed] [Google Scholar]
- 16. Freud A. G., Becknell B., Roychowdhury S., Mao H. C., Ferketich A. K., Nuovo G. J., Hughes T. L., Marburger T. B., Sung J., Baiocchi R. A., Guimond M., Caligiuri M. A. (2005) A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity 22, 295–304 [DOI] [PubMed] [Google Scholar]
- 17. Chantakru S., Wang W. C., van den Heuvel M., Bashar S., Simpson A., Chen Q., Croy B. A., Evans S. S. (2003) Coordinate regulation of lymphocyte-endothelial interactions by pregnancy-associated hormones. J. Immunol. 171, 4011–4019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang J. H., Yamada A. T., Croy B. A. (2009) DBA-lectin reactivity defines natural killer cells that have homed to mouse decidua. Placenta 30, 968–973 [DOI] [PubMed] [Google Scholar]
- 19. Croy B. A., Xie X. (2006) In vivo models for studying homing and function of murine uterine natural killer cells. Methods Mol. Med. 122, 77–92 [DOI] [PubMed] [Google Scholar]
- 20. Croy B. A., Zhang J., Tayade C., Colucci F., Yadi H., Yamada A. T. (2010) Analysis of uterine natural killer cells in mice. In Natural Killer Cell Protocols of Methods in Molecular Biology, Humana, New York, NY, USA, 465–503 [DOI] [PubMed] [Google Scholar]
- 21. Fletcher A. L., Seach N., Reiseger J. J., Lowen T. E., Hammett M. V., Scott H. S., Boyd R. L. (2009) Reduced thymic Aire expression and abnormal NF-κB2 signaling in a model of systemic autoimmunity. J. Immunol. 182, 2690–2699 [DOI] [PubMed] [Google Scholar]
- 22. Ueda Y., Kondo M., Kelsoe G. (2005) Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J. Exp. Med. 201, 1771–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laouar Y., Sutterwala F. S., Gorelik L., Flavell R. A. (2005) Transforming growth factor-β controls T helper type 1 cell development through regulation of natural killer cell interferon-γ. Nat. Immunol. 6, 600–607 [DOI] [PubMed] [Google Scholar]
- 24. Demehri S., Liu Z., Lee J., Lin M. H., Crosby S. D., Roberts C. J., Grigsby P. W., Miner J. H., Farr A. G., Kopan R. (2008) Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol. 6, e123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rochman Y., Spolski R., Leonard W. J. (2009) New insights into the regulation of T cells by γc family cytokines. Nat. Rev. Immunol. 9, 480–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Croy B. A., Guimond M. J., Luross J., Hahnel A., Wang B., van den Heuvel M. (1997) Uterine natural killer cells do not require interleukin-2 for their differentiation or maturation. Am. J. Reprod. Immunol. 37, 463–470 [DOI] [PubMed] [Google Scholar]
- 27. Ashkar A. A., Black G. P., Wei Q., He H., Liang L., Head J. R., Croy B. A. (2003) Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J. Immunol. 171, 2937–2944 [DOI] [PubMed] [Google Scholar]
- 28. Ma A., Koka R., Burkett P. (2006) Diverse functions of IL-2, IL-15 and IL-17 in lymphoid homeostasis. Annu. Rev. Immunol. 24, 657–679 [DOI] [PubMed] [Google Scholar]
- 29. Ye W., Zheng L.-M., Young J., Liu C. (1996) The involvement of interleukin (IL)-15 in regulating the differentiation of granulated metrial gland cells in mouse pregnant uterus. J. Exp. Med. 184, 2405–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trundley A., Moffett A. (2004) Human uterine leukocytes and pregnancy. Tissue Antigens 63, 1–12 [DOI] [PubMed] [Google Scholar]
- 31. Fritz J. H., Gommerman J. L. (2011) Cytokine/stromal cell networks and lymphoid tissue environments. J. Interferon Cytokine Res. 31, 277–289 [DOI] [PubMed] [Google Scholar]
- 32. Crellin N. K., Trifari S., Kaplan C. D., Cupedo T., Spits H. (2010) Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J. Exp. Med. 207, 281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yadi H., Burke S., Madeja Z., Hemberger M., Moffett A., Colucci F. (2008) Unique receptor repertoire in mouse uterine NK cells. J. Immunol. 181, 6140–6147 [DOI] [PubMed] [Google Scholar]
- 34. Anson-Cartwright L., Dawson K., Holmyard D., Fisher S. J., Lazzarini R. A., Cross J. C. (2000) The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat. Genet. 25, 311–314 [DOI] [PubMed] [Google Scholar]
- 35. Burke S. D., Barrette V. F., Bianco J., Thorne J. G., Yamada A. T., Pang S. C., Adams M. A., Croy B. A. (2010) Spiral arterial remodeling is not essential for normal blood pressure regulation in pregnant mice. Hypertension 55, 729–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rochman Y., Kashyap M., Robinson G. W., Sakamoto K., Gomez-Rodriguez J., Wagner K-U., Leonard W. J. (2010) Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc. Natl. Acad. Sci. USA 107, 19455–19460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilson C. B., Rowell E., Sekimata M. (2009) Epigenetic control of T-helper-cell differentiation. Nat. Rev. Immunol. 9, 91–105 [DOI] [PubMed] [Google Scholar]
- 38. Tayade C., Fang Y., Black G. P., V A. P., Jr., Erlebacher A., Croy B. A. (2005) Differential transcription of Eomes and T-bet during maturation of mouse uterine natural killer cells. J. Leukoc. Biol. 78, 1347–1355 [DOI] [PubMed] [Google Scholar]
- 39. Yagi R., Junttila I. S., Wei G., Urban J. F., Jr., Zhao K., Paul W. E., Zhu J. (2010) The transcription factor GATA3 actively represses RUNX3 protein-regulated production of Interferon-γ. Immunity 32, 507–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ashkar A. A., Di Santo J. P., Croy B. A. (2000) Interferon γ contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J. Exp. Med. 192, 259–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Delgado S. R., McBey B. A., Yamashiro S., Fujita J., Kiso Y., Croy B. A. (1996) Accounting for the peripartum loss of granulated metrial gland cells, a natural killer cell population, from the pregnant mouse uterus. J. Leukoc. Biol. 59, 262–269 [PubMed] [Google Scholar]
- 42. Ziegler S. F., Artis D. (2010) Sensing the outside world: TSLP regulates barrier immunity. Nat. Immunol. 11, 289–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ziegler S. F., Liu Y-J. (2006) Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat. Immunol. 7, 709–714 [DOI] [PubMed] [Google Scholar]
- 44. Taylor R. T., Patel S. R., Lin E., Butler B. R., Lake J. G., Newberry R. D., Williams I. R. (2007) Lymphotoxin-independent expression of TNF-related activation-induced cytokine by stromal cells in cryptopatches, isolated lymphoid follicles, and Peyer's patches. J. Immunol. 178, 5659–5667 [DOI] [PubMed] [Google Scholar]
- 45. Kruse A., Merchant M. J., Hallmann R., Butcher E. C. (1999) Evidence of specialized leukocyte-vascular homing interactions at the maternal/fetal interface. Eur. J. Immunol. 29, 1116–1126 [DOI] [PubMed] [Google Scholar]
- 46. Kruse A., Martens N., Fernekorn U., Hallmann R., Butcher E. C. (2002) Alterations in the expression of homing-associated molecules at the maternal/fetal interface during the course of pregnancy. Biol. Reprod. 66, 333–345 [DOI] [PubMed] [Google Scholar]
- 47. Lin Y., Zhong Y., Shen W., Chen Y., Shi J., Di J., Zeng S., Saito S. (2008) TSLP-induced placental DC activation and IL-10+ NK cell expansion: comparative study based on BALB/c × C57BL/6 and NOD/SCID × C57BL/6 pregnant models. Clin. Immunol. 126, 104–117 [DOI] [PubMed] [Google Scholar]
- 48. Kyewski B., Peterson P. (2010) Aire, master of many trades. Cell 140, 24–26 [DOI] [PubMed] [Google Scholar]
- 49. Cohen J. N., Guidi C. J., Tewalt E. F., Qiao H., Rouhani S. J., Ruddell A., Farr A. G., Tung K. S., Engelhard V. H. (2010) Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J. Exp. Med. 207, 681–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.