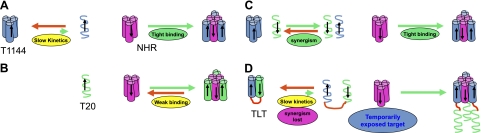
Figure 7.
Putative mechanism of the synergy of the T20-T1144 combination in inhibiting HIV-1 gp41-mediated virus-cell fusion. Stable α-helical structures are shown as cylinders, and unstructured peptides as helix lines; arrows indicate peptide chain direction from N to C terminus. C-peptide fusion inhibitors interact with NHR to form hetero-6-HB to prevent fusogenic 6-HB formation and stop virus-cell fusion processes. Two features in C-peptide-NHR interaction include the temporary exposure of NHR for a couple of minutes and the use of a, d residues of C-peptide fusion inhibitors to interact with NHR and form an external layer of 6-HB where 3 C peptides are separated from each other. A) T1144 forms very stable 6-HB with NHR, which can be considered irreversible binding. However, since its trimer dominates in solution and thus requires dissociation into monomer to expose its a, d position binding sites for effective interaction with NHR, its inhibitory activity is restrained by its slow association rate with the NHR target. B) On the other hand, T20's loose association in solution enables its direct interaction with NHR; however, since the T20/NHR complex is not sufficiently stable and the interaction is reversible, its inhibitory activity is restrained by its weak binding affinity with NHR target. C) In the T20/T1144 combination, loose and transient T20-T1144 pairs are formed as a result of their weak interaction, and the loose T20-T1144 pair rapidly interacts with NHR to form dead-end T1144/NHR 6-HB, unleashing the kinetic restraint of T1144 and causing synergy. D) T20-T1144 interaction in TLT forms a stable structure and shields binding sites of both T20 and T1144; meanwhile, the kinetic benefit gained from the loose and transient interaction between T20 and T1144 is lost, thus abolishing the synergistic anti-HIV-1 effect observed in the T20/T1144 combination.