Abstract
BACKGROUND AND OBJECTIVES:
Extremely low gestational age neonates are more likely than term infants to develop cognitive impairment. Few studies have addressed antenatal risk factors of this condition. We identified antenatal antecedents of cognitive impairment determined by the Mental Development Index (MDI) portion of the Bayley Scales of Infant Development, Second Edition (BSID-II), at 24 months corrected age.
METHODS:
We studied a multicenter cohort of 921 infants born before 28 weeks of gestation during 2002 to 2004 and assessed their placentas for histologic characteristics and microorganisms. The mother was interviewed and her medical record was reviewed. At 24 months adjusted age, children were assessed with BSID-II. Multinomial logistic models were used to estimate odds ratios.
RESULTS:
A total of 103 infants (11%) had an MDI <55, and 99 infants (11%) had an MDI between 55 and 69. No associations were identified between organisms recovered from the placenta and developmental delay. Factors most strongly associated with MDI <55 were thrombosis of fetal vessels (OR 3.1; 95% confidence interval [CI] 1.2, 7.7), maternal BMI >30 (OR 2.0; 95% CI 1.1, 3.5), maternal education ≤12 years (OR 3.4; 95% CI 1.9, 6.2), nonwhite race (OR 2.2; 95% CI 1.3, 3.8), birth weight z score < −2 (OR 2.8; 95% CI 1.1, 6.9), and male gender (OR 2.7; 95% CI 1.6, 4.5).
CONCLUSIONS:
Antenatal factors, including thrombosis of fetal vessels in the placenta, severe fetal growth restriction, and maternal obesity, convey information about the risk of cognitive impairment among extremely premature newborns.
KEY WORDS: prematurity, placenta, chorioamnionitis, fetal growth restriction, mental development
What's Known on this Subject:
Among extremely premature infants, survival has improved, but the rate of cognitive impairment has not. Impaired cognition is the most frequent developmental problem identified in survivors. Several antenatal factors have been associated with cognitive impairment, mostly related to social disadvantage.
What This Study Adds:
In addition to social disadvantage, antenatal characteristics associated with cognitive impairment include maternal obesity and thrombosis of fetal stem vessels. Prenatal infection and inflammation were not associated with impaired early cognitive function among extremely preterm infants.
Among extremely low gestational age newborns (ELGANs), the survival rate improved during the 1990s, but the rate of low scores on assessments of cognition did not.1,2 Cognitive impairment is the most frequent developmental problem identified in extremely premature survivors.3–5 Impaired early cognitive function, assessed with the Bayley Scales of Infant Development (BSID), is moderately predictive of cognitive function at school age.6
Antenatal factors associated with cognitive limitations in ELGANs include unmarried mother,7 minority race,5 fewer years of education,5 lack of antenatal steroid exposure,5,8 maternal smoking during pregnancy,8 and preeclampsia.9 Most previous studies relied on samples defined by birth weight,10–15 which distorts associations with factors that influence birth weight,16 whereas some relied on small samples,9,11,17 and none focused on detailed data about prenatal factors.
METHODS
The ELGAN Study
The ELGAN study was designed to identify characteristics and exposures that increase the risk of structural and functional neurologic disorders in ELGANs.18 During 2002 to 2004, women delivering before 28 weeks of gestation at 1 of 14 participating institutions in 11 cities in 5 states were asked to enroll in the study. The enrollment and consent processes were approved by the individual institutional review boards.
Mothers were approached for consent either on antenatal admission or shortly after delivery, depending on clinical circumstance and institutional preference. A total of 1249 mothers of 1506 infants consented. Approximately 260 women were either missed or did not consent to participate.
Demographic and Pregnancy Variables
After delivery, a trained research nurse interviewed each mother in her native language by using a structured data collection and following procedures contained in a manual. The mother’s report of her own characteristics and exposures, as well as the sequence of events leading to preterm delivery was taken as truth, even when her medical record provided discrepant information.
Shortly after discharge, the research nurse reviewed the maternal chart by using a second structured data collection form to collect information about events after admission. The clinical circumstances that led to each maternal admission and ultimately to each preterm delivery were operationally defined.19 A course of antenatal adrenal corticosteroids given to enhance fetal lung maturation was considered complete if the gravida received 2 doses of betamethasone 24 hours apart and delivered at least 48 hours after the first dose, or if she received 4 doses of dexamethasone at 12-hour intervals. Neonatal data were collected from the newborn’s medical record.
Variable Definitions
The gestational age estimates were based on a hierarchy of the quality of available information. Most desirable were estimates based on the dates of embryo retrieval or intrauterine insemination or fetal ultrasound before the 14th week (62%). When these were not available, reliance was placed sequentially on a fetal ultrasound at 14 or more weeks (29%), last menstrual period without fetal ultrasound (7%), and gestational age recorded in the log of the NICU (1%). The birth weight z score is the number of standard deviations the infant’s birth weight is above or below the median weight of infants at the same gestational age in a standard data set.20 The 6 initiators of preterm delivery, preterm labor, prelabor rupture of the fetal membranes, placental abruption, cervical insufficiency, preeclampsia, and delivery for fetal indications, are defined elsewhere.19
Placentas
All who biopsied the placenta were trained to ensure sterile technique. A piece of the chorion and underlying trophoblast tissue was removed under sterile conditions from the placenta at the midpoint of the longest distance between the cord insertion and the edge of the disk, and then placed in liquid nitrogen until transfer to a –80°C freezer. Eighty-two percent of the placentas were sampled within 1 hour of delivery. The microorganism recovery rate was not influenced by the interval between birth and sampling. The microbiologic procedures are described in detail elsewhere,21 as are the procedures for the preparation and reading of histologic slides.22
Fetal thrombi were recognized when fibrin was seen attached to a vessel wall with ingrowth of endothelial cells. This degree of organization excludes acute thrombi, but there are no accepted criteria for assigning an exact age to these thrombi. Villous injury was not required for the diagnosis of thrombi. Not all thrombi were occlusive. Many, but not all thrombi, were associated with demonstrable villous injury, such as hemorrhage or sclerosis, but villous injury was not explicitly coded.
Level 3 severity of chorionic plate inflammation was defined as >20 neutrophils/20×. Grade 3 inflammation of the amnion was defined as numerous large or confluent foci, whereas grade 4 was defined as necrosis. Inflammation in the chorion/decidua was similarly, but separately, graded.
Inflammation in the umbilical cord was graded from 0 to 5. Grade 3 required neutrophils in perivascular Wharton jelly, grade 4 required panvasculitis and umbilical cord vasculitis extending deep into Wharton jelly, and grade 5 required a “halo lesion” (ring of precipitate in Wharton jelly encircling each vessel). Neutrophilic infiltration into fetal stem vessels in the chorionic plate required that neutrophils appeared to have migrated toward the amnionic cavity.
Developmental Assessment at 24 Months
Families were invited to bring their child for a developmental assessment close to the time when he or she would be 24 months corrected age. Fully 91% of children had this developmental assessment, which included a neurologic examination19,23,24 and the BSID, second edition (BSID-II).25 Of these children, 77% had their examination within the range of 23.5–27.9 months. All BSID-II assessments were age adjusted. Neurologic examiners were asked to rate the child on the Gross Motor Function Classification System (GMFCS)26 separately from the neurologic examination.
Certified examiners administered and scored the BSID-II. Before testing, examiners were told the child's age. After completion of testing, they were told the gestational age so that the unadjusted Mental Development Index (MDI) and Psychomotor Development Index could be obtained. Only 2% of examiners indicated at the time of the examination that they had more than a limited amount of information about the child.
Cognitive impairment was defined as an MDI <70. An MDI <55 was considered severe cognitive impairment.
The child was classified as nontestable on a scale if his or her impairments prohibited standardized administration, or if >2 items were judged to be “not applicable.”
Data Analysis
The generalized form of the null hypothesis that we evaluated states that the risk of a low BSID-II score is not associated with any maternal, pregnancy, or delivery characteristic. To avoid attributing cognition and perception deficits to motor impairments, we limited our analyses to children who were able to walk independently (GMFCS <1), and who, therefore, were unlikely to have functionally important fine-motor impairments.
Because our outcomes of interest (MDI <55 and MDI between 55 and 69) are mutually exclusive and each is appropriately compared with the same referent group (MDI ≥70), we used maximum-likelihood multinomial (polytomous) logistic regression (Stata command: mlogit; Stata Corp, College Station, TX) to calculate odds ratios. We selected variables as potential confounders if identified in the literature or if in our data they were associated with both another relevant exposure and low MDI with probabilities ≤0.25.27
To adjust for potential confounders, we created 2 individual conditional logistic multivariable models, each comparing children in 1 of the 2 abnormal outcome groups to the same referent group (ie, those with MDI ≥70). These models contained a hospital cluster term to account for the possibility that infants born at a particular hospital are more like each other than like infants born at other hospitals.
RESULTS
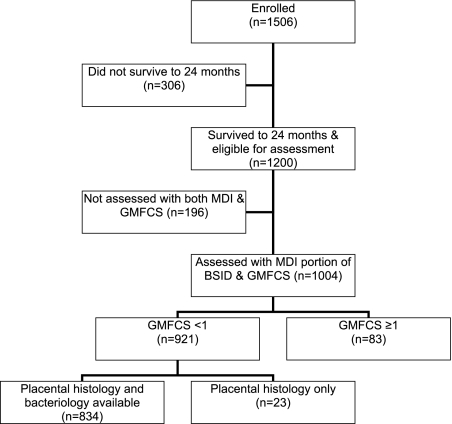
A total of 1200 infants survived to 24 months and 85% of surviving study participants were assessed with BSID-II and GMFCS (Fig 1). After excluding those with impaired gross motor function (GMFCS ≥1), 921 were included in the analyses described in this article. Placenta microbiology and histology were available for 85% (n = 783) of these 921 infants.
FIGURE 1.
Study participants who contributed data to the present analysis among the 1506 newborns enrolled in the Extremely Low Gestational Age Newborn Study.
Maternal Prepregnancy Characteristics
Infants whose mother was black, single, or eligible for public insurance were at increased risk of MDI <55, as were infants whose mother had less than a high school equivalent education, or a prepregnancy BMI of >30 (Table 1).
TABLE 1.
Risks of MDI Scores Associated With Social and Demographic Characteristics of the Mother
| Maternal Characteristics | <55 | 55–69 | ≥70 | Row N | |
|---|---|---|---|---|---|
| Racial identity | White | 7 | 8 | 85 | 545 |
| Black | 20 | 15 | 65 | 242 | |
| Other | 13 | 16 | 71 | 119 | |
| Hispanic | Yes | 17 | 13 | 70 | 108 |
| No | 11 | 10 | 79 | 808 | |
| Age | <21 | 14 | 12 | 74 | 125 |
| 21–35 | 11 | 11 | 78 | 618 | |
| >35 | 10 | 10 | 80 | 178 | |
| Years of education | <12 | 28 | 12 | 60 | 142 |
| 12 (high school) | 11 | 15 | 75 | 232 | |
| >12 to <16 | 8 | 10 | 80 | 201 | |
| 16 (college) | 7 | 6 | 86 | 176 | |
| >16 | 4 | 8 | 88 | 138 | |
| Marital status | Single | 16 | 14 | 70 | 379 |
| Married | 8 | 9 | 83 | 542 | |
| Self and/or partner | Yes | 10 | 11 | 79 | 800 |
| support | No | 20 | 11 | 69 | 101 |
| Public insurance | Yes | 17 | 15 | 69 | 336 |
| No | 8 | 9 | 86 | 566 | |
| Prepregnancy BMI | <18.5 | 9 | 6 | 85 | 66 |
| 18.5 to <25 | 9 | 11 | 80 | 452 | |
| 25 to <30 | 11 | 9 | 80 | 183 | |
| >30 | 16 | 14 | 70 | 184 | |
| Maximum number of infants | 103 | 99 | 719 | 921 | |
| Percent | 11 | 11 | 78 | ||
These are row percents.
Pregnancy Characteristics and Exposures
Of all the factors listed in Table 2, only assisted conception was associated with a decreased risk of MDI <55.
TABLE 2.
Risks of MDI Scores Associated With Pregnancy Characteristics and Exposures During Pregnancy
| Pregnancy Characteristics | <55 | 55–69 | ≥70 | Row N | |
|---|---|---|---|---|---|
| Smoking prepregnancy | Yes | 14 | 12 | 75 | 212 |
| No | 11 | 11 | 79 | 685 | |
| Smoking during pregnancy | Yes | 12 | 15 | 73 | 117 |
| No | 11 | 10 | 79 | 781 | |
| Passive smoking | Yes | 14 | 9 | 77 | 212 |
| No | 11 | 11 | 78 | 683 | |
| Years since last pregnancy | <1 | 8 | 15 | 77 | 107 |
| 1–2 | 14 | 15 | 71 | 144 | |
| 2+ | 15 | 12 | 73 | 267 | |
| Conception assistance | Yes | 6 | 8 | 87 | 197 |
| No | 13 | 12 | 75 | 698 | |
| No. of prenatal visits | 10+ | 9 | 14 | 77 | 619 |
| < 10 | 12 | 10 | 78 | 273 | |
| Vaginal bleeding | Yes | 9 | 11 | 80 | 361 |
| during 1st 12 wk | No | 13 | 11 | 76 | 534 |
| Vaginal bleeding | Yes | 8 | 11 | 81 | 261 |
| after 12 wk | No | 13 | 11 | 77 | 634 |
| Fever during pregnancy | Yes | 9 | 12 | 79 | 58 |
| No | 11 | 11 | 78 | 837 | |
| Vaginal/cervical infection | Yes | 14 | 14 | 72 | 113 |
| No | 11 | 10 | 79 | 782 | |
| Urinary tract infections | Yes | 8 | 13 | 79 | 134 |
| No | 12 | 10 | 75 | 761 | |
| Highest white blood cell counta | >20 K | 9 | 4 | 88 | 187 |
| ≤20 K | 12 | 13 | 75 | 715 | |
| Any medication | Yes | 12 | 11 | 78 | 786 |
| No | 9 | 13 | 78 | 108 | |
| Aspirin | Yes | 15 | 11 | 71 | 46 |
| No | 11 | 11 | 78 | 846 | |
| Nonsteroidal anti-inflammatory drugs | Yes | 9 | 12 | 79 | 57 |
| No | 12 | 11 | 78 | 834 | |
| Acetaminophen | Yes | 9 | 9 | 82 | 453 |
| No | 14 | 12 | 74 | 438 | |
| Antibiotic | Yes | 11 | 13 | 77 | 265 |
| No | 12 | 10 | 78 | 627 | |
| Maximum no. of infants | 103 | 99 | 719 | 921 | |
| Percent | 11 | 11 | 78 | ||
These are row percents.
Within the interval from before delivery to 48 h after delivery.
Delivery Characteristics
The highest risks of MDI <55 were found with shorter duration of labor and when infants were delivered for fetal indications or for maternal preeclampsia, and the lowest risk was found when delivery was due to placental abruption (Table 3).
TABLE 3.
Risks of MDI Scores Associated With Delivery Characteristics
| Delivery Characteristics | <55 | 55–69 | ≥70 | Row N | |
|---|---|---|---|---|---|
| Number of fetuses | 1 | 12 | 10 | 78 | 609 |
| 2+ | 9 | 12 | 79 | 312 | |
| Antenatal steroids | Complete course | 12 | 11 | 77 | 593 |
| Partial course | 9 | 12 | 79 | 234 | |
| None | 11 | 9 | 81 | 94 | |
| Magnesium | For tocolysis | 11 | 10 | 80 | 503 |
| For PE | 15 | 12 | 74 | 121 | |
| No | 10 | 13 | 77 | 288 | |
| Cesarean delivery | Yes | 10 | 10 | 80 | 618 |
| No | 14 | 12 | 74 | 303 | |
| Initiator of delivery | PTL | 9 | 11 | 80 | 414 |
| pPROM | 14 | 9 | 76 | 202 | |
| Preeclampsia | 16 | 11 | 74 | 121 | |
| Abrupt | 5 | 11 | 84 | 101 | |
| Cervical insufficiency | 15 | 10 | 75 | 48 | |
| Fetal indication | 17 | 20 | 63 | 35 | |
| Duration of labor | 0 h | 13 | 12 | 74 | 227 |
| ≤12 h | 17 | 9 | 74 | 211 | |
| >12 h | 8 | 11 | 82 | 483 | |
| Duration of ROM | <1 h | 11 | 11 | 78 | 539 |
| 1–24 h | 10 | 10 | 79 | 144 | |
| >24 h | 12 | 11 | 78 | 238 | |
| Maximum no. of infants | 103 | 99 | 719 | 921 | |
| Percent | 11 | 11 | 78 | ||
These are row percents. preeclampsia (PE),; prolonged preterm rupture of membranes (pPROM),; preterm labor (PTL),; rupture of membranes (ROM).
Placental Cultures and Histologic Findings
Placental inflammation and presence of microorganisms were not associated with low MDI. The only placental finding associated with low MDI was thrombosis of fetal stem vessels; however, there were only 40 infants with this exposure (Tables 4 and 5).
TABLE 4.
Risks of MDI Scores Associated With Organisms Cultured From the Placenta
| Organisms | <55 | 55–69 | ≥70 | Row N | |
|---|---|---|---|---|---|
| Total sample | |||||
| No. of organisms isolated | 0 | 11 | 10 | 79 | 435 |
| 1 | 10 | 11 | 78 | 201 | |
| 2+ | 11 | 11 | 78 | 198 | |
| Any anaerobe | Yes | 8 | 10 | 81 | 224 |
| No | 12 | 11 | 77 | 610 | |
| Any aerobe | Yes | 13 | 11 | 76 | 260 |
| No | 10 | 11 | 79 | 574 | |
| Any Mycoplasma | Yes | 15 | 12 | 72 | 85 |
| No | 10 | 11 | 79 | 749 | |
| Skin organismsa | Yes | 10 | 11 | 79 | 154 |
| No | 11 | 11 | 78 | 680 | |
| Vaginal organismsb | Yes | 10 | 12 | 78 | 132 |
| No | 11 | 11 | 78 | 702 | |
| Maximum no. of infants | 91 | 90 | 653 | 834 | |
| Percent | 11 | 11 | 78 | ||
| Cesarean delivery sample | |||||
|---|---|---|---|---|---|
| No. of organisms isolated | 0 | 11 | 11 | 78 | 333 |
| 1 | 8 | 9 | 83 | 142 | |
| 2+ | 10 | 9 | 81 | 81 | |
| Any anaerobe | Yes | 5 | 10 | 84 | 116 |
| No | 11 | 10 | 79 | 440 | |
| Any aerobe | Yes | 12 | 7 | 81 | 129 |
| No | 9 | 11 | 80 | 427 | |
| Any Mycoplasma | Yes | 10 | 10 | 80 | 41 |
| No | 10 | 10 | 80 | 515 | |
| Skin organismsa | Yes | 7 | 12 | 81 | 69 |
| No | 10 | 10 | 80 | 487 | |
| Vaginal organismsb | Yes | 7 | 11 | 82 | 55 |
| No | 10 | 10 | 80 | 501 | |
| Maximum no. of infants | 55 | 57 | 444 | 556 | |
| Percent | 10 | 10 | 80 | ||
These are row percents.
Corynebacterium sp, Propionebacterium sp, Staphylococcus sp.
Prevotella bivia, Lactobacillus sp, Peptostreptococcus magnus, Gardnerella vaginalis.
TABLE 5.
Risks of MDI Scores Associated With Histologic Characteristics of the Placenta, Total Sample
| Histologic Characteristic | < 55 | 55–69 | ≥70 | Row N | |
|---|---|---|---|---|---|
| Inflammation chorionic platea | Yes | 9 | 12 | 79 | 159 |
| No | 11 | 11 | 78 | 679 | |
| Inflammation external | Yes | 12 | 10 | 78 | 295 |
| membranesb | No | 11 | 12 | 78 | 547 |
| Neutrophil infiltration fetal stem | Yes | 12 | 10 | 78 | 209 |
| vessels | No | 10 | 11 | 78 | 621 |
| Umbilical cord vasculitisc | Yes | 11 | 13 | 76 | 137 |
| No | 11 | 11 | 79 | 682 | |
| Thrombosis of fetal stem vessels | Yes | 23 | 5 | 73 | 40 |
| No | 10 | 11 | 79 | 789 | |
| Infarct | Yes | 9 | 15 | 75 | 142 |
| No | 11 | 10 | 78 | 705 | |
| Increased syncytial knots | Yes | 12 | 14 | 74 | 174 |
| No | 11 | 10 | 79 | 676 | |
| Decidual hemorrhage/ | Yes | 11 | 12 | 77 | 129 |
| Fibrin deposition | No | 11 | 10 | 78 | 708 |
| Maximum no. of placentas | 94 | 94 | 669 | 857 | |
| Percent | 11 | 11 | 78 | ||
These are row percents.
Stage 3 and severity 3.
Grades 3 and 4.
Grades 3, 4, and 5.
Infant Characteristics
The risk of MDI <55 decreased with increasing gestational age, birth weight, birth weight z score, and head circumference z score. Boys were also at moderately higher risk than girls of MDI <55 (Table 6).
TABLE 6.
Risks of MDI Scores Associated With Characteristics of the Infants
| Characteristics of the Infant | <55 | 55–69 | ≥70 | Row N | |
|---|---|---|---|---|---|
| Gender | Male | 15 | 13 | 72 | 472 |
| Female | 8 | 8 | 84 | 449 | |
| Gestational | 23–24 | 15 | 11 | 74 | 163 |
| Age, wk | 25–26 | 12 | 10 | 78 | 435 |
| 27 | 8 | 11 | 80 | 323 | |
| Birth weight, g | ≤750 | 14 | 13 | 73 | 318 |
| 751–1000 | 11 | 10 | 79 | 421 | |
| >1000 | 5 | 10 | 85 | 182 | |
| Birth weight | < –2 | 22 | 14 | 68 | 51 |
| z scorea | ≥ –2, < –1 | 15 | 12 | 73 | 123 |
| ≥ –1 | 10 | 10 | 80 | 747 | |
| Head | < –2 | 21 | 10 | 70 | 73 |
| circumference | ≥ –2, < –1 | 14 | 14 | 72 | 207 |
| z scorea | ≥ –1 | 9 | 18 | 81 | 609 |
| Maximum number of infants | 103 | 99 | 719 | 921 | |
| Percent | 11 | 11 | 78 | ||
These are row percents.
Birth weight and head circumference z scores based on Yudkin et al20 standard.
Multivariable Analyses
In multivariable models that included the total sample, heightened risk of MDI <55 was predicted by nonwhite race, maternal education <12 years, maternal BMI >30, male gender, and birth weight z score >2 SDs below the mean. When the sample was limited to children for whom we had information about histologic characteristics, the same variables were predictive of MDI <55, whereas thrombosis of fetal vessels provided additional information about increased risk (odds ratio 3.1, 95% confidence interval [CI] 1.2, 7.7). For both total sample and the histology-limited sample, the only significant risk factors for MDI 55 to 69 were male and nonwhite race. Multinomial models without a hospital cluster term provided results that were very similar to those provided by individual logistic regression models that included a hospital cluster term (Table 7).
TABLE 7.
Multivariable Analysis of Variables Associated With Low MDI
| Variables | <55 | 55–69 |
|---|---|---|
| Total sample (n = 921) | ||
| Gestational age 23–24 wk | 1.9 (0.97, 3.6) | 1.0 (0.5, 1.9) |
| Gestational age 25–26 wk | 1.2 (0.7, 2.1) | 0.8 (0.5, 1.3) |
| Single mother | 1.3 (0.7, 2.2) | 1.2 (0.7, 2.1) |
| BMI >30 | 1.9 (1.1, 3.1)a | 1.3 (0.9, 2.3) |
| Vaginal/cervical infection | 1.2 (0.6, 2.2) | 1.4 (0.8, 2.6) |
| Cesarean delivery | 0.8 (0.5, 1.2) | 0.8 (0.5, 1.4) |
| BWZ < –2 | 2.2 (1.5, 7.5)a | 1.9 (0.7, 4.8) |
| Male | 2.5 (1.6, 4.1)a | 2.0 (1.3, 3.2)a |
| Nonwhite race | 2.3 (1.4, 3.8)a | 2.1 (1.3, 3.5)a |
| Mother's education <12 y | 2.8 (1.6, 4.9)a | 0.9 (0.5, 1.8) |
| Mother's education >16 y | 0.8 (0.4, 1.5) | 0.7 (0.4, 1.2) |
| Total histology/BSID sample (n = 857) | ||
| Gestational age 23–24 wk | 1.9 (0.9, 3.9) | 1.1 (0.6, 2.1) |
| Gestational age 25–26 wk | 1.1 (0.6, 2.0) | 0.8 (0.4, 1.3) |
| Single mother | 1.2 (0.7, 2.2) | 1.2 (0.7, 2.0) |
| BMI >30 | 2.0 (1.1, 3.5)a | 1.2 (0.7, 2.1) |
| Vaginal/cervical infection | 1.2 (0.6, 2.4) | 1.4 (0.7, 2.7) |
| Cesarean delivery | 0.9 (0.5, 1.5) | 0.9 (0.5, 1.5) |
| BWZ < –2 | 2.8 (1.1, 6.9)a | 1.9 (0.7, 5.0) |
| Male | 2.7 (1.6, 4.5)a | 2.1 (1.3, 3.4)a |
| Thrombosis fetal vessels | 3.1 (1.2, 7.7)a | 0.6 (0.1, 2.5) |
| Increased syncytial knots | 1.4 (0.8, 2.5) | 1.5 (0.9, 2.5) |
| Nonwhite race | 2.2 (1.3, 3.8)a | 2.2 (1.3, 3.7)a |
| Mother's education <12 y | 3.4 (1.9, 6.2)a | 1.1 (0.6, 2.2) |
| Mother's education >16 y | 0.7 (0.4, 1.4) | 0.7 (0.4, 1.3) |
Risk ratios and 95% CIs. The referent group is infants with MDI ≥70. BWZ, birthweight z score.
p < .05.
Discussion
Three maternal attributes (obesity, nonwhite race, and maternal education) and 1 placental finding (thrombosis of fetal stem vessels) were associated with impaired early cognitive function in extremely preterm infants. Neonatal predictors of such impairment were birth weight z score < –2 (ie, below the third centile) and male gender. In general, stronger associations were found for MDI <55 than for MDI 55 to 69.
Our finding that male gender was predictive of impaired early cognitive function agrees with most5,28 but not all29 studies of extremely preterm infants.28,30,31 The mechanisms for this association are not well understood. Boys born prematurely have a greater risk of morbidities, such as cerebral white matter damage and chronic lung disease, and gender differences in brain structure and response to injury might also be important.32
In our multivariable regression models, fetal growth restriction is a predictor of an MDI <55, while not predicting a less severely low MDI. Others, too, have found that growth-restricted infants are at increased risk of cognitive limitations.33,34 One explanation invokes placental dysfunction, which can restrict placental transfer of substrates for brain development.35 An alternative explanation is that fetal growth restriction increases the risk of neonatal complications36,37 that are more proximally related to cognitive impairment.38
Children who experience a major postnatal infection (including bacteremia and necrotizing enterocolitis) are more likely than others to have cerebral white matter injury39 and cognitive impairment.15,17 Perinatal inflammation, as evidenced by chorioamnionitis, is also a risk factor for white matter injury and cerebral palsy.40 We, however, did not find any evidence that antenatal inflammation predicts cognitive impairment.
The only histologic characteristic of the placenta associated with cognitive impairment was thrombosis of fetal stem vessels, which is considered a lesion of uteroplacental circulation and has been associated with adverse neonatal outcomes.41 In addition to stillbirth,42 perinatal liver disease,43 and intrauterine growth restriction in monochorionic twins,44 fetal vessel thrombosis has been linked to adverse neurologic outcomes, including neonatal encephalopathy45 and neonatal stroke.46 In the ELGAN cohort, it has been associated with ventriculomegaly of the offspring,47 which, in turn, is associated with low MDI.4
One antecedent that we identified, maternal obesity, is of particular interest because it is potentially modifiable and its prevalence is increasing.48 Although maternal obesity is predictive of higher maternal blood levels of C-reactive protein49 and interleukin-6,50 we found no indirect evidence to support a link between maternal inflammation, as indicated by placental findings and initiators of preterm delivery, and subsequent impaired infant cognitive function. Maternal obesity is associated with multiple complications of pregnancy51 and with perinatal mortality.52–55 Perhaps most relevant to our study is the association with fetal growth restriction.19,56 In addition, obesity is linked to chronic illnesses57,58 and social disadvantage,59 which might affect the mother-infant interaction and the resources available to enhance the home environment.
The association of assisted conception with a low MDI, seen in univariable analyses, was no longer significant in multivariable analyses. The most likely explanation is that the risk information provided by the assisted conception variable is more strongly conveyed by 2 other correlates of socioeconomic status: race and education.
We are not aware of any other large prospective studies relating early cognitive functioning in extremely preterm infants to placental findings or initiators of preterm delivery. A potential limitation of this study is that MDI <55 is only moderately predictive of cognitive impairment at school age.60 The strengths of this prospective study include the large number of infants, selection of participants based on gestational age rather than birth weight, and evaluations by individuals who were not aware of infants’ medical or social histories.
This study has implications for efforts to prevent cognitive impairment among extremely premature infants. One antecedent identified here, maternal obesity, is potentially modifiable during the preconception period and pregnancy. The prevalence of another antecedent, fetal growth restriction, could be decreased by interventions to prevent severe preeclampsia, which occurred in 13% of our sample of extremely preterm infants.
To what extent the cognitive adversities associated with fetal growth restriction reflect postnatal phenomena (especially those associated with chronic lung disease) remains unclear. Postnatal disorders appear to be involved in explaining some of the relationship between chronic lung disease and impaired development.7 If the occurrence of these disorders can be minimized, then there is hope that the influence of fetal growth restriction on cognitive deficiencies can be reduced.
CONCLUSIONS
Antenatal characteristics associated with impaired mental development include maternal BMI >30, nonwhite race, maternal education, thrombosis of fetal stem vessels, and male gender. Our findings do not support an important role of prenatal infection or inflammation in the development of impaired early cognitive function among extremely preterm infants.
Acknowledgments
Participating institutions (site principal investigators, pathologists, and developmentalists): Baystate Medical Center, Springfield, MA (Bhavesh Shah, Solveg Pflueger, Susan McQuiston, Herbert Gilmore, Karen Christianson); Beth Israel Deaconess Medical Center, Boston, MA (Camilia R. Martin, Jonathan Hecht, Alaska Morgan, Haim Bassan, Cecil Hahn, Samantha Butler, Adre Duplessis, Colleen Hallisey); Brigham & Women's Hospital, Boston, MA (Linda J. Van Marter); Children’s Hospital, Boston, MA (Alan Leviton); Massachusetts General Hospital, Boston, MA (Robert Insoft, Drucilla Roberts, Kalpathy Krishnamoorthy, Maureen Quill); Floating Hospital for Children at Tufts Medical Center, Boston, MA (Cynthia Cole/John Fiascone, Ina Bhan, Cecelia Keller, Karen Miller, Page Church, Caitlyn Hurley); University of Massachusetts Memorial Health Center, Worcester, MA (Francis Bednarek, Gamze Ayata, Robin Adair, Alice Miller, Rick Bream, Albert Scheiner, Beth Powers); Yale-New Haven Hospital, New Haven, CT (Richard Ehrenkranz, Miguel Reyes-Múgica, Eduardo Zambrano, Vinita Parkas, Cindy Miller, Elaine Romano, Nancy Close, Linda Mayes, Joanne Williams); Wake Forest University Baptist Medical Center and Forsyth Medical Center, Winston-Salem, NC (T. Michael O’Shea, Dennis W. Ross, Gail Hounshell, Don Goldstein, Lisa Washburn, Cherrie Heller, Robert Dillard, Debbie Hiatt, Deborah Allred); University Health Systems of Eastern Carolina, Greenville, NC (Stephen Engelke, John Christie, Kathyrn Kerkering, Steve Engelke, Lynn Whitley, Rebecca Helms, Peter Resnik); North Carolina Children's Hospital, Chapel Hill, NC (Carl Bose, Chad Livasy, Diane Marshall, Lisa Bostic, Janice Wereszczak, Mandy Taylor, Carol Torres, Kristi Milowic, Ginny Bose); DeVos Children's Hospital, Grand Rapids, MI (Mariel Portenga, Barbara Doss, Lynn Fagerman, Steve Pasynrnak, Victoria Caine, Wendy Burdo-Hartman, Dianah Sutton); Sparrow Hospital, Lansing, MI (Padmani Karna); Michigan State University, East Lansing, MI (Nigel Paneth, Madeleine Lenski, Nicholas Olomu, Padu Karna, Victoria Caine, Joan Price); University of Chicago Hospital, Chicago, IL (Michael D. Schreiber, Aliya Husain, Sunila O'Connor, Michael Msall, Susan Plesha-Troyke, Leslie Caldarelli, Grace Yoon); William Beaumont Hospital, Royal Oak, MI (Daniel Batton, Chung-ho Chang, Karen Brooklier, Katie Solomon, Dan Batton, Melisa Oca, Beth Kring); and pathologists not at participating sites (Harvey Kliman, Pat Senagore).
The authors gratefully acknowledge the contributions of our subjects and their families, as well as those of our colleagues.
Glossary
- BSID-II
Bayley Scales of Infant Development, second edition
- CI
confidence interval
- ELGAN
extremely low gestational age newborn
- GMFCS
Gross Motor Function Classification System
- MDI
Mental Development Index
- OR
odds ratio
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by a cooperative agreement with The National Institute of Neurologic Disorders and Stroke (NINDS) (5U01NS040069-05). Funded by the National Institutes of Health (NIH).
References
- 1.Doyle LW, Victorian Infant Collaborative Study Group Neonatal intensive care at borderline viability—is it worth it? Early Hum Dev. 2004;80(2):103–113 [DOI] [PubMed] [Google Scholar]
- 2.Washburn LK, Dillard RG, Goldstein DJ, Klinepeter KL, Jackson BG, O'Shea TMD. Survival and developmental impairment in extremely low gestational age newborns born 1980-2000. Pediatr Res. 2002;51(4):288A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuban K, Allred E, O'Shea TM, et al. Cranial ultrasound lesions in the NICU predict cerebral palsy at age 2 years in children who were born at extremely low gestational age. J Child Neurol. 2008;24:63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Shea TM, Kuban KC, Allred EN, et al. Extremely Low Gestational Age Newborns Study Investigators Neonatal cranial ultrasound lesions and developmental delays at 2 years of age among extremely low gestational age children. Pediatrics. 2008;122(3). Available at: www.pediatrics.org/cgi/content/full/122/3/e662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR, EPICure Study Group The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2005;90(2):F134–F140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts G, Anderson PJ, Doyle LW, Victorian Infant Collaborative Study Group The stability of the diagnosis of developmental disability between ages 2 and 8 in a geographic cohort of very preterm children born in 1997. Arch Dis Child. 2010;95(10):786–790 [DOI] [PubMed] [Google Scholar]
- 7.Laughon M, O'Shea TM, Allred EN, et al. Chronic lung disease and the risk of developmental delay at two years of age in children born before 28 weeks’ gestation. Pediatrics. 2009;124(2):637–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiechl-Kohlendorfer U, Ralser E, Pupp Peglow U, Reiter G, Trawöger R. Adverse neurodevelopmental outcome in preterm infants: risk factor profiles for different gestational ages. Acta Paediatr. 2009;98(5):792–796 [DOI] [PubMed] [Google Scholar]
- 9.Cheng SW, Chou HC, Tsou KI, Fang LJ, Tsao PN. Delivery before 32 weeks of gestation for maternal pre-eclampsia: neonatal outcome and 2-year developmental outcome. Early Hum Dev. 2004;76(1):39–46 [DOI] [PubMed] [Google Scholar]
- 10.Schmidt B, Roberts RS, Davis P, et al. Caffeine for Apnea of Prematurity Trial Group Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357(19):1893–1902 [DOI] [PubMed] [Google Scholar]
- 11.Bozynski ME, Nelson MN, Matalon TA, et al. Prolonged mechanical ventilation and intracranial hemorrhage: impact on developmental progress through 18 months in infants weighing 1200 grams or less at birth. Pediatrics. 1987;79(5):670–676 [PubMed] [Google Scholar]
- 12.Vohr BR, Poindexter BB, Dusick AM, et al. National Institute of Child Health and Human Development National Research Network Persistent beneficial effects of breast milk ingested in the neonatal intensive care unit on outcomes of extremely low birth weight infants at 30 months of age. Pediatrics. 2007;120(4). Available at: www.pediatrics.org/cgi/content/full/120/4/e953 [DOI] [PubMed] [Google Scholar]
- 13.Whitaker A, Johnson J, Sebris S, et al. Neonatal cranial ultrasound abnormalities: association with developmental delay at age one in low birth weight infants. J Dev Behav Pediatr. 1990;11(5):253–260 [PubMed] [Google Scholar]
- 14.Ehrenkranz RA, Walsh MC, Vohr BR, et al. National Institutes of Child Health and Human Development Neonatal Research Network Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353–1360 [DOI] [PubMed] [Google Scholar]
- 15.Stoll BJ, Hansen NI, Adams-Chapman I, et al. National Institute of Child Health and Human Development Neonatal Research Network Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365 [DOI] [PubMed] [Google Scholar]
- 16.Arnold CC, Kramer MS, Hobbs CA, McLean FH, Usher RH. Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. Am J Epidemiol. 1991;134(6):604–613 [DOI] [PubMed] [Google Scholar]
- 17.Shah DK, Doyle LW, Anderson PJ, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr 2008;153(2):170–175 [DOI] [PubMed]
- 18.O’Shea TM, Allred EN, Dammann O, et al. ELGAN study Investigators The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85(11):719–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McElrath TF, Hecht JL, Dammann O, et al. ELGAN Study Investigators Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. 2008;168(9):980–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yudkin PL, Aboualfa M, Eyre JA, Redman CWG, Wilkinson AR. New birthweight and head circumference centiles for gestational ages 24 to 42 weeks. Early Hum Dev. 1987;15(1):45–52 [DOI] [PubMed] [Google Scholar]
- 21.Onderdonk AB, Delaney ML, DuBois AM, Allred EN, Leviton A, Extremely Low Gestational Age Newborns (ELGAN) Study Investigators Detection of bacteria in placental tissues obtained from extremely low gestational age neonates. Am J Obstet Gynecol. 2008;198(1):110–, e1–e7. [DOI] [PubMed] [Google Scholar]
- 22.Hecht JL, Allred EN, Kliman HJ, et al. Elgan Study Investigators Histological characteristics of singleton placentas delivered before the 28th week of gestation. Pathology. 2008;40(4):372–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuban KC, Allred EN, O’Shea M, Paneth N, Pagano M, Leviton A, ELGAN Study Cerebral Palsy-Algorithm Group An algorithm for identifying and classifying cerebral palsy in young children. J Pediatr. 2008;153(4):466–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuban KC, O’Shea M, Allred E, et al. Video and CD-ROM as a training tool for performing neurologic examinations of 1-year-old children in a multicenter epidemiologic study. J Child Neurol. 2005;20(10):829–831 [DOI] [PubMed] [Google Scholar]
- 25.Bayley N. Bayley Scales of Infant Development. 2nd ed. San Antonio, TX: Psychological Corporation; 1993 [Google Scholar]
- 26.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223 [DOI] [PubMed] [Google Scholar]
- 27.Dales LG, Ury HK. An improper use of statistical significance testing in studying covariables. Int J Epidemiol. 1978;7(4):373–375 [DOI] [PubMed] [Google Scholar]
- 28.Hintz SR, Kendrick DE, Vohr BR, Kenneth Poole W, Higgins RD, Nichd Neonatal Research Network Gender differences in neurodevelopmental outcomes among extremely preterm, extremely-low-birthweight infants. Acta Paediatr. 2006;95(10):1239–1248 [DOI] [PubMed] [Google Scholar]
- 29.Beaino G, Khoshnood B, Kaminski M, et al. EPIPAGE Study Group Predictors of the risk of cognitive deficiency in very preterm infants: the EPIPAGE prospective cohort. Acta Paediatr. 2011;100(3):370–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shankaran S, Johnson Y, Langer JC, et al. Outcome of extremely-low-birth-weight infants at highest risk: gestational age < or =24 weeks, birth weight < or =750 g, and 1-minute Apgar < or =3. Am J Obstet Gynecol. 2004;191(4):1084–1091 [DOI] [PubMed] [Google Scholar]
- 31.Stoelhorst GM, Rijken M, Martens SE, et al. Leiden Follow-Up Project on Prematurity Developmental outcome at 18 and 24 months of age in very preterm children: a cohort study from 1996 to 1997. Early Hum Dev. 2003;72(2):83–95 [DOI] [PubMed] [Google Scholar]
- 32.Johnston MV, Hagberg H. Sex and the pathogenesis of cerebral palsy. Dev Med Child Neurol. 2007;49(1):74–78 [DOI] [PubMed] [Google Scholar]
- 33.Leitner Y, Fattal-Valevski A, Geva R, et al. Neurodevelopmental outcome of children with intrauterine growth retardation: a longitudinal, 10-year prospective study. J Child Neurol. 2007;22(5):580–587 [DOI] [PubMed] [Google Scholar]
- 34.Leitner Y, Fattal-Valevski A, Geva R, et al. Six-year follow-up of children with intrauterine growth retardation: long-term, prospective study. J Child Neurol. 2000;15(12):781–786 [DOI] [PubMed] [Google Scholar]
- 35.Reuss ML, Paneth N, Susser M. Does the loss of placental hormones contribute to neurodevelopmental disabilities in preterm infants? Dev Med Child Neurol. 1994;36(8):743–747 [PubMed] [Google Scholar]
- 36.Bose C, Van Marter LJ, Laughon M, et al. Extremely Low Gestational Age Newborn Study Investigators Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics. 2009;124(3). Available at: www.pediatrics.org/cgi/content/full/124/3/e450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engineer N, Kumar S. Perinatal variables and neonatal outcomes in severely growth restricted preterm fetuses. Acta Obstet Gynecol Scand. 2010;89(9):1174–1181 [DOI] [PubMed] [Google Scholar]
- 38.Anderson PJ, Doyle LW. Neurodevelopmental outcome of bronchopulmonary dysplasia. Semin Perinatol. 2006;30(4):227–232 [DOI] [PubMed] [Google Scholar]
- 39.Glass HC, Bonifacio SL, Chau V, et al. Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics. 2008;122(2):299–305 [DOI] [PubMed] [Google Scholar]
- 40.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. 2000;284(11):1417–1424 [DOI] [PubMed] [Google Scholar]
- 41.Redline RW, Pappin A. Fetal thrombotic vasculopathy: the clinical significance of extensive avascular villi. Hum Pathol. 1995;26(1):80–85 [DOI] [PubMed] [Google Scholar]
- 42.Saleemuddin A, Tantbirojn P, Sirois K, et al. Obstetric and perinatal complications in placentas with fetal thrombotic vasculopathy. Pediatr Dev Pathol. 2010;13(6):459–464 [DOI] [PubMed] [Google Scholar]
- 43.Dahms BB, Boyd T, Redline RW. Severe perinatal liver disease associated with fetal thrombotic vasculopathy. Pediatr Dev Pathol. 2002;5(1):80–85 [DOI] [PubMed] [Google Scholar]
- 44.Chan MP, Hecht JL, Kane SE. Incidence and clinicopathologic correlation of fetal vessel thrombosis in mono- and dichorionic twin placentas. J Perinatol. 2010;30(10):660–664 [DOI] [PubMed] [Google Scholar]
- 45.McDonald DG, Kelehan P, McMenamin JB, et al. Placental fetal thrombotic vasculopathy is associated with neonatal encephalopathy. Hum Pathol. 2004;35(7):875–880 [DOI] [PubMed] [Google Scholar]
- 46.Elbers J, Viero S, MacGregor D, DeVeber G, Moore AM. Placental pathology in neonatal stroke. Pediatrics. 2011;127(3). Available at: www.pediatrics.org/cgi/content/full/127/3/e722 [DOI] [PubMed] [Google Scholar]
- 47.McElrath TF, Allred EN, Boggess KA, et al. ELGAN Study Investigators Maternal antenatal complications and the risk of neonatal cerebral white matter damage and later cerebral palsy in children born at an extremely low gestational age. Am J Epidemiol. 2009;170(7):819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in pre-pregnancy obesity in nine states, 1993-2003. Obesity (Silver Spring). 2007;15(4):986–993 [DOI] [PubMed] [Google Scholar]
- 49.Madan JC, Davis JM, Craig WY, et al. Maternal obesity and markers of inflammation in pregnancy. Cytokine. 2009;47(1):61–64 [DOI] [PubMed] [Google Scholar]
- 50.Roberts KA, Riley SC, Reynolds RM, et al. Placental structure and inflammation in pregnancies associated with obesity. Placenta. 2011;32(3):247–254 [DOI] [PubMed] [Google Scholar]
- 51.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113(10):1126–1133 [DOI] [PubMed] [Google Scholar]
- 52.Chu SY, Kim SY, Lau J, et al. Maternal obesity and risk of stillbirth: a metaanalysis. Am J Obstet Gynecol. 2007;197(3):223–228 [DOI] [PubMed] [Google Scholar]
- 53.Kristensen J, Vestergaard M, Wisborg K, Kesmodel U, Secher NJ. Pre-pregnancy weight and the risk of stillbirth and neonatal death. BJOG. 2005;112(4):403–408 [DOI] [PubMed] [Google Scholar]
- 54.Leddy MA, Power ML, Schulkin J. The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol. 2008;1(4):170–178 [PMC free article] [PubMed] [Google Scholar]
- 55.Vasudevan C, Renfrew M, McGuire W. Fetal and perinatal consequences of maternal obesity. Arch Dis Child Fetal Neonatal Ed 2011;96(5):F378–F382 [DOI] [PubMed]
- 56.Sebastián Manzanares G, Angel Santalla H, Irene Vico Z, López Criado MS, Alicia Pineda L, José Luis Gallo V. Abnormal maternal body mass index and obstetric and neonatal outcome. J Matern Fetal Neonatal Med. 2011; Early online,1–5 [DOI] [PubMed] [Google Scholar]
- 57.Jungheim ES, Moley KH. Current knowledge of obesity’s effects in the pre- and periconceptional periods and avenues for future research. Am J Obstet Gynecol. 2010;203(6):525–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Messier SP. Obesity and osteoarthritis: disease genesis and nonpharmacologic weight management. Rheum Dis Clin North Am. 2008;34(3):713–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown A, Siahpush M. Risk factors for overweight and obesity: results from the 2001 National Health Survey. Public Health. 2007;121(8):603–613 [DOI] [PubMed] [Google Scholar]
- 60.Hack M, Taylor HG, Drotar D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics. 2005;116(2):333–341 [DOI] [PubMed] [Google Scholar]