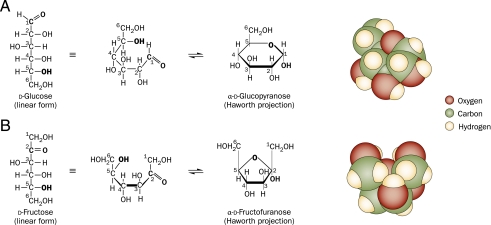
FIGURE 3.
Molecular renditions of (A) glucose and (B) fructose, in the linear, chair, and space-occupying projections. In the linear form, both glucose and fructose possess a reactive aldehyde or one ketone moiety, which can bind nonenzymatically to freely available amino groups of proteins. At normal body temperature and pH, the chair form of glucose predominates. This conformation is a glucopyranose (6-membered ring), with equatorial hydroxyl groups and is molecularly stable, which limits its protein reactivity. However, the chair form of fructose is a fructofuranose (5-membered ring) with 2 axial hydroxymethyl groups that exert allosteric and ionic forces on the unstable furanose ring, which favors the linear form. Thus, at normal body temperature and pH, the majority of fructose exists in the linear form and is more reactive with proteins than is glucose. (Reproduced from Lim et al140.)