Figure 5.
Biological Properties of CCaMKFN-ED and the CaM Binding Assay Using Mutated CCaMK Proteins.
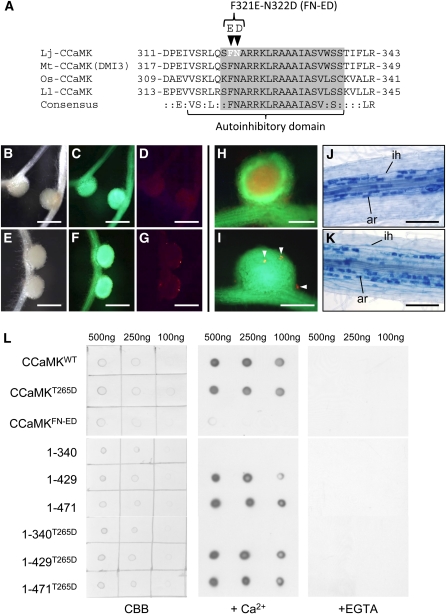
(A) Amino-acid alignment of the CaMBD/autoinhibitory domain of four plant CCaMK proteins. Lj, L. japonicus; Mt, M. truncatula; Os, O. sativa; Ll, L. longiflorum. The CaMBD and autoinhibitory domains assigned by Ramachandiran et al. (1997) are indicated by the gray box and underline, respectively. Arrowheads above the alignment indicate the residues in which mutations (F321E and N322D) were introduced.
(B) to (G) Nodulation of ccamk-3/CCaMKFN-ED roots in the absence ([B] to [D]) and presence ([E] to [G]) of M. loti, observed as bright-field images (B) and (E) and fluorescence images of GFP ([C] and [F]) and DsRed ([D] and [G]).
(H) and (I) RN formed on ccamk-3/CCaMKWT (H) and ccamk-3/CCaMKFN-ED roots (I), shown as merged images of fluorescent field of GFP and DsRed. Arrowheads indicate microcolonies formed on ccamk-3/CCaMKFN-ED nodule.
(J) and (K) Mycorrhization of ccamk-3 roots transformed with CCaMKWT (J) or CCaMKFN-ED (K). ar, arbuscule; ih, intracellular hyphae.
(L) CaM binding assay of CCaMKWT, CCaMKT265D, CCaMKFN-ED (Top) and a deletion series of CCaMK proteins (Bottom). Images are (left) staining of spotted CCaMK proteins with Coomassie brilliant blue (CBB) and immunodetection of the binding of biotin-labeled CaM in the presence (middle) and absence (right) of Ca2+ (+EGTA). The concentrations of the CCaMK proteins spotted on membrane are shown above the images.
Bars in (B) to (G) = 1 mm; bars in (H) and (I) = 500 μm; bars in (J) and (K) = 200 μm.