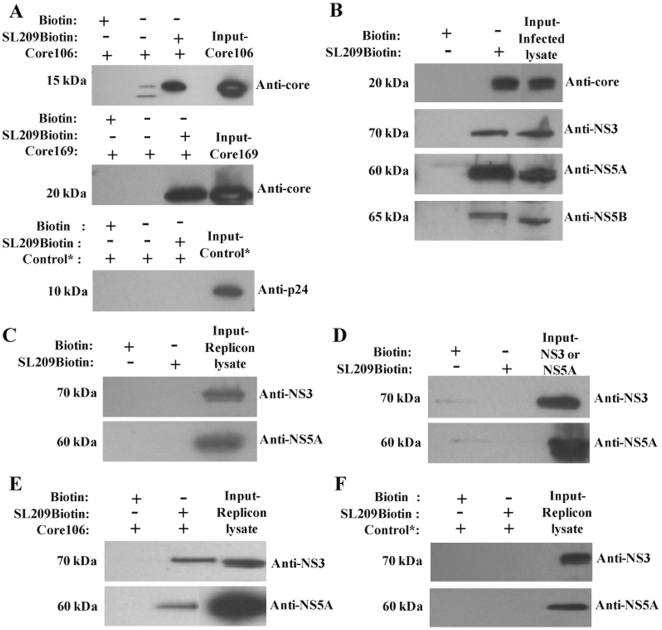
Figure 3. Affinity isolation on SL209-biotin captures native and recombinant HCV core and core-associated proteins.
SL209-biotin was immobilized on streptavidin agarose beads and incubated with core106 (10 µg) or core169 (10 µg) proteins or lysates of Huh-7.5 cells infected with HCV J6/JFH-1. The retained proteins were examined by immuno-blotting using anti-core, NS3, NS5A or NS5B antibodies. One-twentieth of the cell lysate used in the assay was immuno-blotted for all proteins as the input control. As a control, the lysates were incubated with Biotin immobilized on streptavidin agarose beads. As an additional control, a non-specific protein, CTD (C-terminal domain of HIV capsid protein, p24) was incubated with immobilized SL209-biotin. A: SL209-biotin captures/co-precipitates recombinant core106 (top panel) and core169 proteins (middle panel) and does not capture non-specific control protein CTD (bottom panel). B: SL209-biotin captures/co-precipitates core, NS3, NS5A, and NS5B from HCV-infected Huh-7.5 cells, but C: not from replicon containing Huh-7.5 cell lysates. D: SL209-biotin does not co-precipitate recombinant NS3 and NS5A proteins. E: Core protein added to replicon-containing Huh-7.5 cell lysates rescues co-precipitation of NS3 and NS5A. F: CTD protein added to replicon-containing Huh-7.5 cell lysates does not rescue the co-precipitation of NS3 and NS5A.