Abstract
Introduction:
The aim of this prospective, double-blinded study was to investigate the effects of clonidine in co-administration with bupivacaine during spinal anesthesia, regarding the onset and regression of motor and sensory block, postoperative analgesia and possible side effects.
Methods:
We randomly selected 66 male patients (age 35 to 70), from the American Society of Anesthesiologists (ASA) class I–II; these patients were scheduled for transurethral surgical procedures. These patients were randomly allocated into two groups of 33 patients each: group B (bupivacaine) only received 0.5% isobaric bupivacaine 7.5 mg intrathecally and group BC (bupivacaine + clonidine) received bupivacaine 7.5 mg and clonidine 25 μg intrathecally. We performed the spinal anesthesia at a level of L3–L4 with a 25-gauge needle. We assessed the sensory block with a pin-prick, the motor block using the Bromage scale, analgesia with the visual analog scale and sedation with the modified Wilson scale. We also recorded the hemodynamic and respiratory parameters.
Results:
The groups were demographically similar. The mean time of achievement of motor block (Bromage 3) and sensory block at level T9 was significantly shorter in the BC group compared with B group (p = 0.002, p = 0.000, respeectively). The motor block regression time was not significantly different between the two groups (p = 0.237). The postoperative analgesia requirement was significantly longer in group BC compared with group B (p = 0.000). No neurological deficit, sedation or other significant adverse effects were recorded.
Conclusion:
The intrathecal application of clonidine in combination with bupivacaine improves the duration and quality of spinal anesthesia; it also provides longer duration of postoperative analgesia, without significant side effects.
Introduction
Spinal anesthesia has been widely used for urologic operations, particularly in transurethral surgical procedures, because it permits early recognition of symptoms caused by overhydration, transurethral resection of prostate syndrome and bladder perforation.
Smaller doses of intrathecal bupivacaine will reduce the number of blocked dermatomes and decrease the duration of spinal anesthesia; the co-administration of clonidine reduces the dose of bupivacaine and improves the quality of spinal anesthesia.
The antinociceptive properties of clonidine indicate that it might be useful as an alternative to intrathecal opioids for postoperative analgesia,1 thus avoiding the main adverse effects, such as respiratory depression, pruritus and urinary retention. The intrathecal application of clonidine increases the duration of both sensory and motor block,2–5 as well as postoperative analgesia.6 The mechanism of clonidine in spinal anesthesia is reported to be mediated by presynaptic (inhibition of transmitter release)7 and postsynaptic (enhancing hyperpolarization)8,9 effects.
Marked decrease in arterial blood pressure was observed with 75 μg of intrathecal clonidine (in combination with intrathecal morphine),10 whereas relative hemodynamic stability was maintained with doses ≥150 μg, as demonstrated by using clonidine as the sole analgesic.11
The aim of this prospective, randomized, double-blinded study was to evaluate the effects of clonidine in co-administration with bupivacaine during spinal anesthesia, regarding the onset and regression of motor block, sensory block, postoperative analgesia and possible side effects.
Methods
The hospital ethics committee of the University Clinical Centre of Kosovo approved the study and we obtained written informed consent from all patients. We randomly selected 66 male patients (age 35 to 70), American Society of Anesthesiologists (ASA) I–II who were scheduled for transurethral surgical procedures. We randomly allocated them into two groups (B [bupivacaine] and BC [bupivacaine + clonidine], with 33 patients in each group. Patients in group B received intrathecally 0.5% isobaric bupivacaine 7.5 mg and patients in group BC received 0.5% isobaric bupivacaine 7.5 mg plus 0.0015% clonidine 25 μg. These solutions were diluted with 0.9% saline solution to a total volume of 3.5 mL and were prepared by someone not involved in the patients’ care. The anesthesiologist and the patients were blinded to the study solutions.
Patients taking α-adrenergic receptor antagonists, angiotensin-converting enzyme (ACE) inhibitors or calcium channel blockers were excluded from the study. We also excluded patients with psychiatric illness, neurologic disease, a body weight of >120 kg, a height of <150 cm, as well as patients belonging to the class ASA III–V and E.
Patients were not premedicated due to the evaluation of the potential sedation caused by clonidine. Before intrathecal injection, patients underwent standard monitoring (Datex-Ohmeda S/5 (TM) Monitor, Helsinki, Finland), including an electrocardiogram (5 lead), noninvasive blood pressure and pulse oximeter; we also noted baseline vital parameters. We obtained intravenous (IV) access with an 18-gauge IV canula (Novomed Ltd, Dublin, Ireland) and we administered 0.9% sodium chloride solution (250 mL). Spinal anesthesia was performed with the patient in the sitting position, using a 25-gauge Quincke needle (Yale TM Spinal Becton Dickinson, Madrid, Spain) with a midline approach at L3–4 interspace. After intrathecal injection, patients were immediately placed in the supine position and, when T10 sensory block was achieved, they were placed in the lithotomy position for the start of the surgical intervention.
Heart rate and non-invasive arterial blood pressure were measured at 5- to 15-minute intervals, whereas peripheral oxygen saturation was monitored continuously by pulse oximeter during induction, surgery and recovery, and thereafter every hour up to 8 hours, followed by 4-hour intervals up to 24 hours.
During surgery, 0.9% sodium chloride solution was infused at a rate of 8 mL/kg/h. Additional IV fluids (crystalloids, colloids and blood) were administered as perioperatively dictated by blood loss and hemodynamic instability. The blood loss of >500 mL was replaced with maximally 1000 mL of colloids or by packed red blood cells if hemoglobin was <90 g/L. We defined clinically relevant hemodynamic instability as a decrease of 30% or more in mean arterial pressure from baseline value; we treated these patients with 300 mL of additional fluids or incremental IV bolus of 5 mg ephedrine (Claris Lifesciences, Inc., Gujarat, India) if there were unresponsive within 5 minutes.
Clinically relevant bradycardia was defined as a decrease in heart rate of <50/min or a 20% decrease from initial value and was treated with 0.5 mg atropine IV, as needed (Sterop SA Laboratories, Brussels, Belgium). Respiratory depression was defined as a respiratory rate less than 8 breaths/min or a SpO2 less than 85% on room air; oxygen was administered via a face mask (2–4 L\min) if the pulse oximeter read below 90%.
The extension of sensory block was determined by a pin-prick test in the midclavicular line bilaterally. All determinations of the sensory levels were based on a dermatomes chart (Astra Pharmaceutica AG Zürich, Switzerland). The time to achieve the highest level of sensory block (TASB) T10 and the time to regression of sensory block (TRSB) S1 were recorded. Motor block was assessed using the Modified Bromage Scale (0: no motor block; 1: inability to raise extended legs; 2: inability to flex knees, and 3: inability to flex ankle joints). Time to achieve motor block (TAMB) Bromage 3 and time to regression of motor block (TRMB) Bromage 0 were also recorded. The intensity of pain was assessed using a 10-cm visual analog scale (VAS; 0: no pain and 10: worst imaginable pain). Sensory and motor block were assessed every 2 minutes for 15 minutes after intrathecal injection and every 5 minutes thereafter until sensory block regressed to S1.
Sedation was assessed according to the Modified Wilson Sedation Scale ranging from 0 to 4 (1: oriented; 2: drowsy; 3: rousable to mild physical stimulation; and 4: unrousable to mild physical stimulation).
We recorded adverse effects, such as hypotension, bradycardia, respiratory depression, nausea, vomiting, pruritus, sweating and shivering, and possible complications, such as transitory neurologic syndrome, headache, low back pain and cardiac arrest. All data collection was performed by people not involved in patient care. Patients were observed until the level of sensory block was S1 and the motor block was completely resolved (TRMB) (Bromage score: 0); patients were then discharged from the recovery room.
Statistical analysis
The results were analyzed using statistical software (Minitab 14, State College PA). Descriptive statistics are for all continuous variables denoted as mean (minimum-maximum) and ± standard deviation (SD). The comparisons for testing significant differences between the two groups BC and B (for continuous variables) were performed by using Student’s t-test. While, Chi-square test (χ2) was used for categorical variables, such as ASA-Physical Status (PS) (I/II ratio). Statistical significance was set at p < 0.05.
Results
A total 66 patients were studied. There were no significant differences between the two groups with respect to age, height, weight and ASA status (Table 1).
Table 1.
Demographic and baseline characteristics of patients
| BC group (N=33) | B group (N=33) | p | |
|---|---|---|---|
| Age (years) | 59.64 (42–70) ±8.98 | 59.24 (42–70) ±8.86 | 0.856 |
| Weight (kg) | 90.18 (77–106) ±8.09 | 86.24 (60–103) ±10.74 | 0.097 |
| Height (cm) | 172.85 (163–180) ±4.74 | 173.97 (158–182) ±5.22 | 0.365 |
| ASA-PS (I/II ratio) | 21/12 | 22/11 | 0.796 |
BC: intrathecal bupivacaine + clonidine; B: intrathecal bupivacaine alone; ASA-PSL: American Society of Anesthesiologists Physical Status. Values shown as number (N) of patients, mean (min-max) ± standard deviation.
We compared values (mean ± SD) of observed parameters regarding the onset of motor block, onset of sensory block, regression of motor block and regression of sensory block (Table 2).
Table 2.
Comparisons of the follow up parameters between groups
| BC group (N=33) | B group (N=33) | p | |
|---|---|---|---|
| TAMB | 8.273 (5.000–12.000) ±1.941 | 9.697 (7.000–13.000) ±1.551 | 0.002 |
| TASB | 11.394 (8.000–15.000) ±2.150 | 15.758 (13.000–20.000) ±1.562 | 0.000 |
| TRMB | 236.48 (180.00–310.00) ±35.32 | 226.06 (162.00–280.00) ±35.65 | 0.237 |
| TRSB | 434.1 (265.0–521.0) ±78.3 | 263.97 (195.00–325.00) ±40.38 | 0.000 |
BC: intrathecal bupivacaine + clonidine; B: intrathecal bupivacaine alone; TAMB: time to achieve motor block Bromage 3 (minutes); TASB: time to achieve sensory block T10 (minutes); TRMB: time to regression of motor block Bromage 0 (minutes); TRSB: time to regression of sensory block to S1 (minutes). Values shown as number (N) of patients, mean (min-max) ± standard deviation.
A complete motor blockade of the lower extremities (Bromage 3) and sensory block of T9 were observed in all patients. The difference in time of achievement of complete motor block between groups was statistically significant (8.273 ± 1.941 min. in group BC vs. 9.697 ± 1.551 min. in group B), in favour of clonidine group (p = 0.002). We also observed that the mean time to achieve sensory block of T10 was significantly shorter in the clonidine group (11.394 ± 2.150 min.) than in the plain bupivacaine group (15.758 ± 0.562 min.) (p =0.000).
There were no statistically significant differences between the groups in the complete regression of motor block (Bromage scale 0); 236.48 ± 35.32 minutes versus 226.06 ± 35.65 minuties in the BC and B groups, respectively (p = 0.237). The regression of sensory block to S1, the first request for supplemental analgesia (VAS scores at >2 to 3 cm) was significantly longer in the BC group than in the B group (434.1 ± 78. 3 min. vs. 263.97 ± 40.38 min., respectively, p0.000).
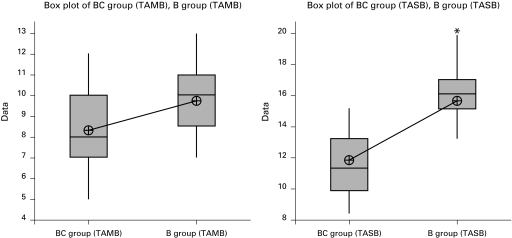
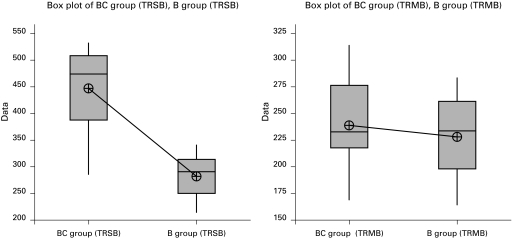
The box plots provide useful information for comparing BC and B group in terms of TAMB, TASB, TRSB and TRMB. There is considerable spread in the median of TAMB and TASB, with group B having the higher median and group BC the lower median (Fig. 1). Additionally, we can observe that there is an outlier for TASB in group B. Unlike Fig. 1, Fig. 2 illustrates a higher median of TRSB in group BC compared to group B. The resulting box plots for TRMB in Fig. 2 do not illustrate significant differences in the median of group BC and B.
Fig. 1.
Box plots of data between BC (intrathecal bupivacaine + clonidine) and B (intrathecal bupivacaine alone) group in terms of TAMB (time to achieve motor block Bromage 3 [minutes]) and TASB (time to achieve sensory block T10 [minutes]).
Fig. 2.
Box plots of data between BC and B group in terms of TRSB (time to regression of sensory block to S1 [minutes]) and TRMB (time to regression of motor block Bromage 0 [minutes]).
There was low incidence of side effects and no patient suffer from complications, such as transitory neurologic syndrome, headache, low back pain and cardiac arrest. The incidence of hypotension was 3 (9%) patients in the clonidine groups compared with 2 (6%) in the plain bupivacaine group. There were no events of bradycardia and respiratory depression in any of the treatment groups. Adverse effects, such as sedation, nausea, vomiting, pruritus, sweating and shivering, were not observed.
Discussion
Our results showed that the low dose of intrathecal clonidine co-administered with a low dose of bupivacaine increased the onset of motor block, onset and duration of sensory block (from time of first request for supplemental analgesia), without delaying motor block in patients undergoing transurethral surgical procedures.
Clonidine is a selective partial agonist for α-2 adrenergic receptors; the analgesic effect following its intrathecal administration is mediated spinally through the activation of postsynaptic α-2 receptors in substantia gelatinosa of the spinal cord.12,13
Many previous studies have used intrathecal clonidine combined with opioids and local anesthetics for labour analgesia and orthopedic surgery,14–16 but not for urologic surgery, particularly transurethral surgical procedures. Gautier and colleagues recommend 15 to 45 μg of clonidine as optimal for supplementing spinal anesthesia;17 in keeping within this range, we chose 25 μg as optimal.
Clonidine (15–30 μg) significantly prolongs sensory blockade and improves postoperative analgesia for gynecological operations,18 knee arthroscopy17 and ambulatory inguinal herniorrhaphy.19 The data match with our results concerning the duration of sensory block-postoperative analgesia. Our results showed that the addition of a small dose (25 μg) of clonidine increased the spread (onset-T9) and duration of sensory block, thereby prolonging postoperative analgesia.
According to some previous studies, intrathecal clonidine alone, even at doses above 450 μg, does not cause muscular weakness and motor blockade,11 but combined with local anesthetics it significantly enhances the intensity and duration of motor blockade.2,4 In our study, however, we found a significant difference in the TAMB between the two groups, in favour of the clonidine group, but we failed to achieve statistical significance in the duration of the motor block.
The higher doses of clonidine have been reported to cause important decreases in arterial pressure and marked sedation.11,20,21 However, as our results demonstrate, a small dose of intrathecal clonidine is not usually associated with systemic side effects, such as bradycardia, hypotension or sedation.17
Relative hemodynamic stability was maintained in both groups. Patients receiving intrathecal clonidine showed small variations in hemodynamic variables which were not clinically relevant. In orthopedic patients, some studies show that changes are not observed in hemodynamic variables with 150 μg.17 Relative cardiovascular stability has been reported with 75 to 100 μg,21 and a significantly decrease of blood pressure is reported with 75 μg intrathecal clonidine (in combination with 0.5 mg intrathecal morphine).10 Therefore, hemodynamic stability in our study can be attributed to a low dose of clonidine.
Sedation, a central effect of α-2 adrenergigcs, may occur after either systemic, epidural or intrathecal administration of clonidine,22,23 notably in the dose range of 150 to 450 μg.11 However, in our study sedation is not observed in any of the patients in BC group, which also could be explained by the use of a low dose of intrathecal clonidine.
We have not noted any significant adverse effects (e.g., respiratory depression, vomiting, nausea, pruritus, shivering, sweating) or complications (e.g., cardiac arrest, postdural puncture headache, backache, transitory neurologic syndrome) as recorded in previous studies.17,22
Conclusion
This study showed that a small-dose of intrathecal clonidine (25 μg) as adjuvant to small-dose (7.5 mg) bupivacaine increased the onset and duration of analgesia and produced an effective spinal anesthesia in patients undergoing transurethral surgical procedures.
We suggest this adjuvant especially for ambulatory anesthesia because it prolongs postoperative analgesia, but not motor block.
Footnotes
Competing interests: None declared.
This paper has been peer-reviewed.
References
- 1.Yaksh TL, Reddy SV. Studies in the primate on the analgetic effects associated with intrathecal actions of opiates, alpha-adrenergic agonists and baclofen. Anesthesiology. 1981;54:451–67. doi: 10.1097/00000542-198106000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Racle JP, Benkhadra A, Poy JY, et al. Prolongation of isobaric bupivacaine spinal anesthesia with epinephrine and clonidine for hip surgery in the elderly. Anesth Analg. 1987;66:442–6. doi: 10.1213/00000539-198705000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Dobrydnjov I, Samarutel J. Enhancement of intrathecal lidocaine by addition of local and systemic clonidine. Acta Anaesthesiol Scand. 1999;43:556–62. doi: 10.1034/j.1399-6576.1999.430512.x. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet F, Buisson VB, Francois Y, et al. Effects of oral and subarachnoid clonidine on spinal anesthesia with bupivacaine. Reg Anesth. 1990;15:211–4. [PubMed] [Google Scholar]
- 5.Gentili ME, Mamelle JC, Le Foll G. Combination of low-dose bupivacaine and clonidine for unilateral spinal anesthesia in arthroscopic knee surgery. Reg Anesth. 1995;20:169–70. [PubMed] [Google Scholar]
- 6.Dobrydnjov I, Axelsson K, Samarutel J, et al. Postoperative pain relief following intrathecal bupivacaine combined with intrathecal or oral clonidine. Acta Anaesthesiol Scand. 2002;46:806–14. doi: 10.1034/j.1399-6576.2002.460709.x. [DOI] [PubMed] [Google Scholar]
- 7.Gordh T, Jr, Jansson I, Hartvig P, et al. Interactions between noradrenergic and cholinergic mechanisms involved in spinal nociceptive processing. Acta Anaesthesiol Scand. 1989;33:39–47. doi: 10.1111/j.1399-6576.1989.tb02857.x. [DOI] [PubMed] [Google Scholar]
- 8.Gaumann DM, Brunet PC, Jirounek P. Clonidine enhances the effects of lidocaine on C-fiber action potential. Anesth Analg. 1992;74:719–25. doi: 10.1213/00000539-199205000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Erne-Brand F, Jirounek P, Drewe J, et al. Mechanism of antinociceptive action of clonidine in nonmyelinated nerve fibres. Eur J Pharmacol. 1999;383:1–8. doi: 10.1016/S0014-2999(99)00620-2. [DOI] [PubMed] [Google Scholar]
- 10.Grace D, Bunting H, Milligan KR, et al. Postoperative analgesia after co-administration of clonidine and morphine by the intrathecal route in patients undergoing hip replacement. Anesth Analg. 1995;80:86–91. doi: 10.1097/00000539-199501000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Filos KS, Goudas LC, Patroni O, et al. Hemodynamic and analgesic profile after intrathecal clonidine in humans: a dose-response study. Anesthesiology. 1994;81:591–601. doi: 10.1097/00000542-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Reddy SVR, Yaksh TL. Spinal noradrenergic terminal system mediates antinociception. Brain Res. 1980;189:391–401. doi: 10.1016/0006-8993(80)90099-2. [DOI] [PubMed] [Google Scholar]
- 13.Brandt SA, Livingston A. Receptor changes in spinal cord of sheep associated with exposure to chronic pain. Pain. 1990;42:323–9. doi: 10.1016/0304-3959(90)91145-9. [DOI] [PubMed] [Google Scholar]
- 14.Mercier FJ, Dounas M, Bouaziz H, et al. The effect of adding a minidose of clonidine to intrathecal sufentanil for labour analgesia. Anesthesiology. 1998;89:594–601. doi: 10.1097/00000542-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Sites BD, Christopher R, Biggs R, et al. Intrathecal clonidine added to a bupivacaine-morphine spinal improves postoperative analgesia following total knee arthroplasty. Anesthesiology. 2002;96:A918. doi: 10.1213/01.ANE.0000055651.24073.59. [DOI] [PubMed] [Google Scholar]
- 16.De Kock M, Gauthier P, Fanord L. Intrathecal ropivacaine and clonidine for ambulantory knee arthroscopy. A dose response study. Anesthesiology. 2001;94:574–8. doi: 10.1097/00000542-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Gautier PE, De Kock M, Luc F, et al. Intrathecal clonidine combined with sufentanil for labour analgesia. Anesthesiology. 1998;88:651–6. doi: 10.1097/00000542-199803000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Juliao MC, Lauretti GR. Low-dose intrathecal clonidine combined with sufentanil as analgesic drugs in abdominal gynecological surgery. J Clin Anesth. 2000;12:357–62. doi: 10.1016/S0952-8180(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 19.Dobrydnjov I, Axelsson K, Thorn SE, et al. Clonidine combined with small-dose bupivacaine during spinal anesthesia for inguinal herniorrhaphy: a randomized double-blinded study. Anesth Analg. 2003;96:1496–503. doi: 10.1213/01.ANE.0000061110.62841.E9. [DOI] [PubMed] [Google Scholar]
- 20.Pan PM, Huang CT, Wei TT, et al. Enhancement of analgesic effect of intrathecal neostigmine and clonidine on bupivacaine spinal anaesthesia. Reg Anesth Pain Med. 1998;23:49–56. doi: 10.1016/s1098-7339(98)90110-9. [DOI] [PubMed] [Google Scholar]
- 21.Fogarty DJ, Carabine UA, Milligan KR. Comparison of the analgesic effects of intrathecal clonidine and intrathecal morphine after spinal anaesthesia in patients undergoing total hip replacement. Br J Anaesth. 1993;71:661–4. doi: 10.1093/bja/71.5.661. [DOI] [PubMed] [Google Scholar]
- 22.Eisenach JC, De Kock M, Klimscha W. Alpha(2)-adrenergic agonists for regional anaesthesia. A clinical review of clonidine (1984–1995) Anesthesiology. 1996;85:655–74. doi: 10.1097/00000542-199609000-00026. [DOI] [PubMed] [Google Scholar]
- 23.Filos KS, Goudas LC, Patroni O, et al. Intrathecal clonidine as a sole analgesic for pain relief after cesarean section. Anesthesiology. 1992;77:267–74. doi: 10.1097/00000542-199208000-00008. [DOI] [PubMed] [Google Scholar]