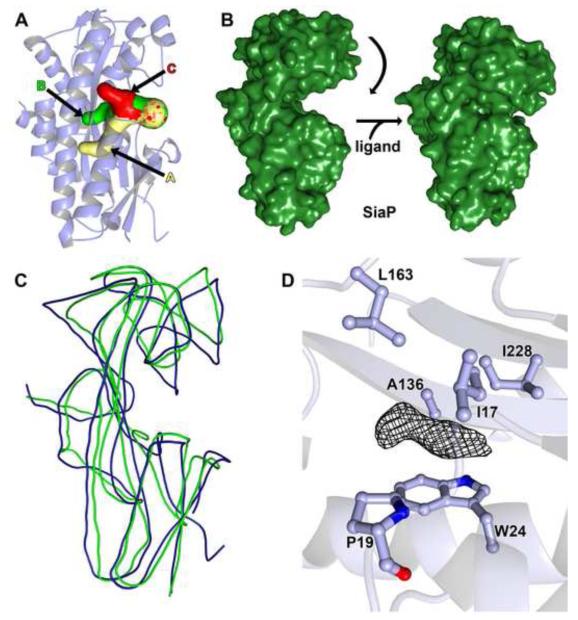
Figure 8. Features of the Tp0957 cleft.
(A) Cavities proximal to the Tp0957 cleft. Cavity A is shown in cream, Cavity B in green, and Cavity C in red, and the three cavities are also labeled. All three fuse near to the surface of the protein; the mottled appearance of the surface here demonstrates that all three of the calculated paths contribute to this opening. The secondary structure of Tp0957 is shown semi-transparently for clarity. The orientation is slightly different from that drawn in Fig. 7. The surfaces were calculated using Caver 2.0 71. (B) Observed closure of SiaP 37. The left side shows the unliganded form of SiaP, in the analogous orientation to that shown for Tp0957 in Fig. 7B. Upon ligand binding, the upper lobe moves down (right side), enveloping the ligand. (C) Superposition of Tp0957 and SiaP (open form). Both structures are shown as smoothed traces through their respective Cα atoms; Tp0957 is colored blue, and SiaP is colored green. (D) Unassigned electron density in Tp0957’s cleft. A 2mFo-DFc map contoured at the 1-σ level is shown for the density. Nearby side chains are shown and labeled. The secondary structure of Tp0957 is shown semi-transparently for clarity.