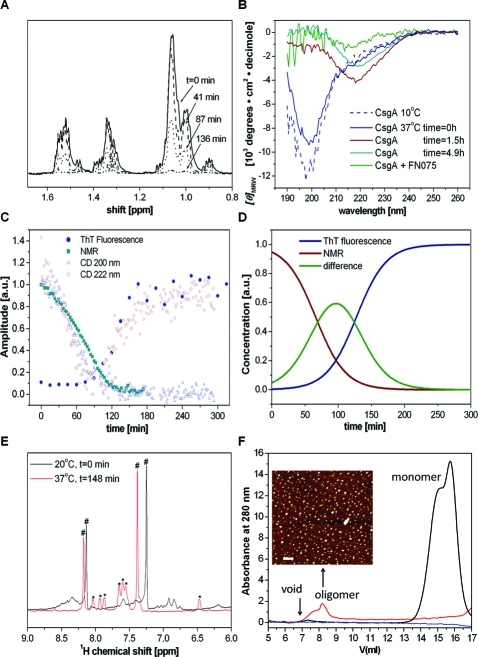
Figure 2.
Time-dependent structural conversion of CsgA from monomer to β-rich polymer. (A) Depletion of monomeric CsgA during the fibrillation reaction probed by 1H NMR at 37 °C. The figure is an expansion of the aliphatic region in one-dimensional spectra. (B) Time-dependent far-UV CD acquired at 37 °C. The spectrum at 10 °C is a reference of monomeric CsgA; fibrillation is induced by a temperature jump to 37 °C (4.5 μM CsgA and in one sample also 45 μM FN075). (C) Overlay of kinetic traces from NMR, CD, and ThT fluorescence. (D) Sigmodial fits of monomer depletion (from NMR) and fiber build-up (from ThT). The analysis reveals the presence of an intermediate during the reaction. (E) 1H NMR expanded in the amide region upon FN075 addition to CsgA (450 μM FN075, 45 μM CsgA): (∗) FN075 peaks, (#) imidazole (small amount leftover from purification). (F) Size exclusion chromatograms of CsgA in the absence and presence of FN075. Ten micromolar freshly isolated (black) or overnight incubated protein (at room temperature) with 100 μM (red) or without (blue) FN075. Inset: AFM of the oligomer fraction (red trace) (bar 200 nm).