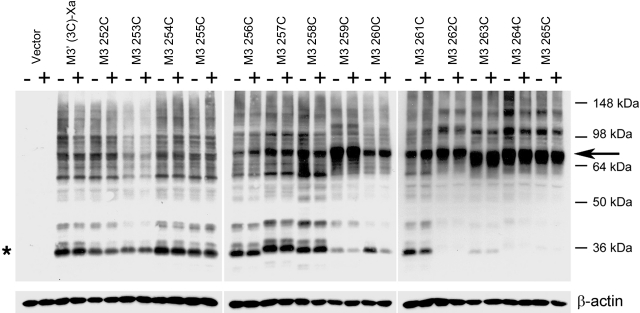
Figure 2.
Detection of disulfide cross-linked mutant M3R homodimers via Western blotting. Membranes prepared from COS-7 cells expressing the indicated Cys-substituted mutant M3Rs were incubated with 100 μM Cu-Phen in the absence (−) or presence (+) of 1 mM carbachol. Membrane extracts were then subjected to SDS-PAGE under nonreducing conditions. Receptor proteins were visualized via Western blotting analysis using the polyclonal anti-M3R antibody. Putative receptor monomers (asterisk) corresponding to functional cell surface receptors can be detected at ∼40 kDa (24). The ∼80 kDa bands (arrow) are predicted to correspond to cross-linked receptor dimers. Similar results were obtained in 2 to 4 independent Western blotting experiments.