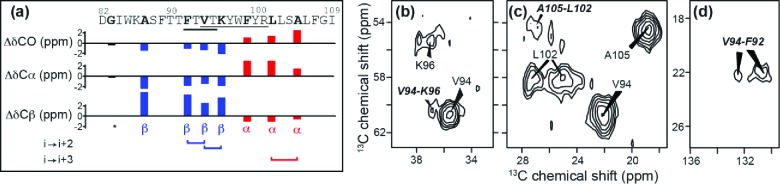
Figure 5.
(a) SSNMR indicates α-helical conformation (red) for labeled sites in the IMD, while most CSD residues have a β-conformation (blue). Residues of the CRAC motif (V94–Y100) span the helix–strand boundary, and the protein binding motif (F92–T95) is in a β-strand. The CSI shows the difference (Δδ) of C′, Cα, and Cβ chemical shifts from random coil values. Black bars lack a defined secondary structure (e.g., G83). Also indicated are the observations of i → i + 2 contacts between F92–V94 and V94–K96 (b, d) and an i → i + 3 contact between L102–A105 (c). The spectra are from long-distance 13C–13C experiments with 400 ms PDSD mixing, obtained at 600 MHz 1H frequency and 8 kHz MAS at 283 K.