Figure 4.
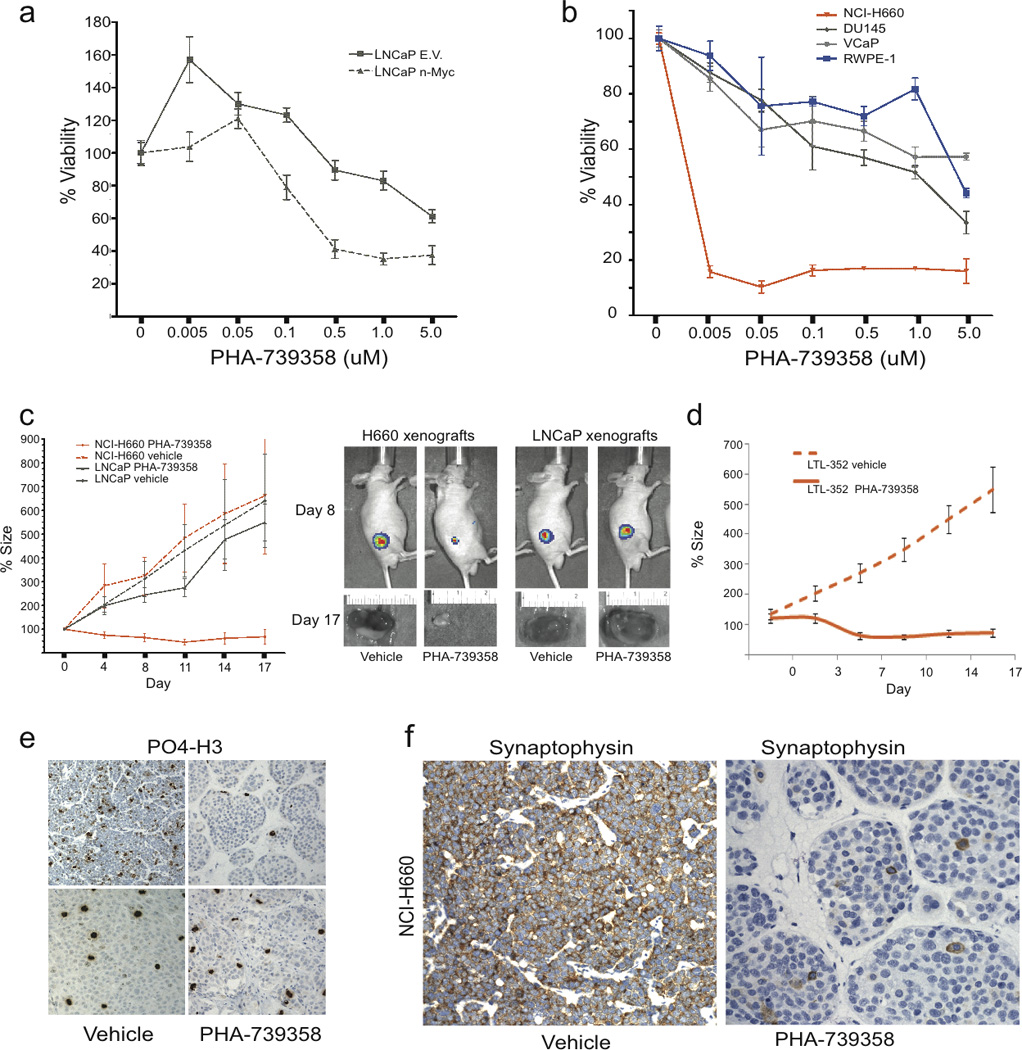
NEPC demonstrates enhanced sensitivity to Aurora Kinase Inhibitor therapy compared to PCA (A) Viability assay of LNCaP cells transfected with MYCN or Empty Vector (EV) at 72 hours after treatment with vehicle or indicated doses of the pan-Aurora kinase inhibitor PHA-739358. (B) Viability assay of RWPE (blue circles), VCaP (gray diamonds), DU145 (gray triangles), and NCI-H660 (orange triangles) at 72 hours after treatment with vehicle or indicated doses of PHA-739358. (C) Percent tumor size after treatment of LNCaP (gray) and NCI-H660 (red) xenografts with vehicle (dotted lines) or PHA-739358 30 mg/kg IP BID (solid lines) twice a day for 5 days relative to day 0. Luciferase imaging at day 8 and tumor photographs at day 17 of representative tumors following treatment with either vehicle or PHA-73935. (D) Percent tumor size after treatment of LTL-362 xenografts with vehicle (dotted lines) or PHA-739358 30 mg/kg IP BID (solid lines) twice a day for 5 days relative to day 0. (E) Immunohistochemical staining for phosphorylated histone 3 (PO4-H3) in NCI-H660 or LNCaP tumors at day 4 of treatment with either vehicle or PHA-739358. (F) Immunohistochemistry for the neuroendocrine marker, synaptophysin, in NCI-H660 xenografts treated with vehicle (positive) and PHA-739358 (negative).
