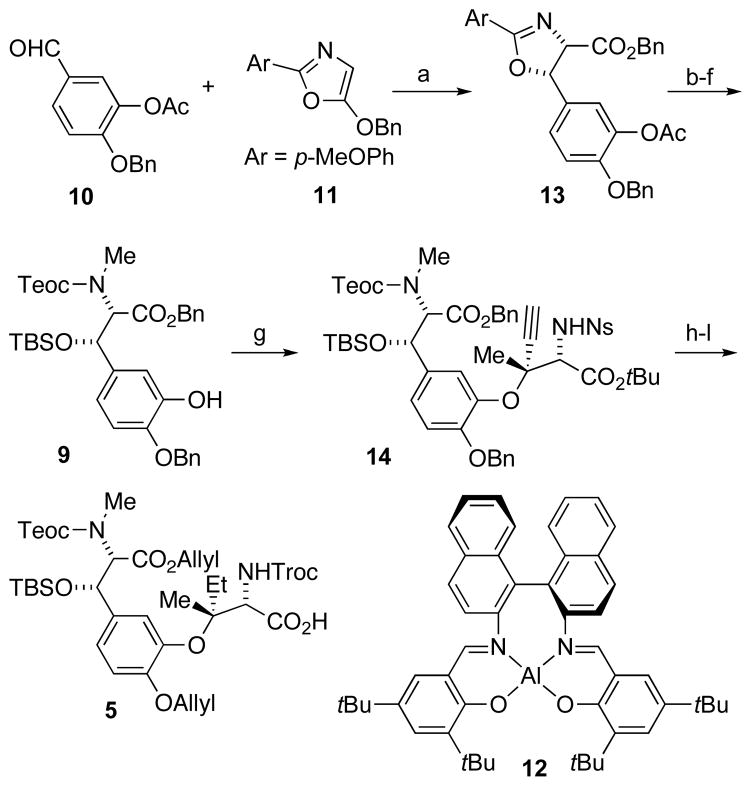
Scheme 3.
Reagents and conditions: a) Na2SO4, 12, AgSbF6, 3 Å MS, PhCH3, 25 h, 96%, 98% ee; b) MeOTf, CH2Cl2, 2 h then NaBH4, NaHCO3, H2O, 0 °C, 15 min, 72%; c) 0.1 N (COOH)2, THF, 24 h, 99%; d) TBSOTf, 2,6-lutidine, CH2Cl2, 0 °C, 30 min, 87%; e) Teoc-Cl, NaHCO3, CH3CN, 16 h, 98%; f) NH2NH2·H2O, THF, 0 °C, 3 h, 98%; g) 8, CuOAc, DBU, PhCH3, 0 °C, 12 d, 87%; h) PhSH, Cs2CO3, DMF, 1 h, 92%; i) 2,2,2-trichloroethyl chloroformate, Na2CO3, THF, 1 h, 96%; j) H2, 20% Pd(OH)2/C, EtOAc, 4 d, 99%; k) allyl bromide, DBU, DMF, 5 h, 70%; l) SiO2, PhCH3, 90 °C, 20 h. DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene; DMF = N,N-dimethylformamide; MS = molecular sieves; Tf = trifluoromethanesulfonate.