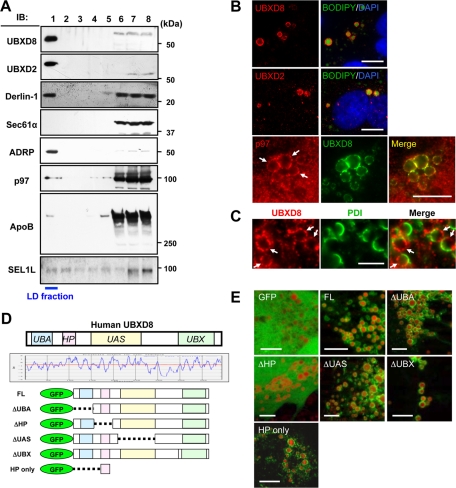
FIGURE 1:
UBXD8 and other ERAD-related proteins were present in LDs of Huh7 cells. The cells were maintained in the normal culture medium without any fatty acid supplement in this and subsequent experiments unless otherwise stated. (A) Fractions obtained by sucrose density-gradient ultracentrifugation were analyzed by Western blotting. LDs were recovered in the fraction of the lowest density (fraction 1), which was verified by enrichment of ADRP. In addition to UBXD8, UBXD2, and p97, which were identified by mass spectrometric analysis, Derlin-1 and SEL1L were also found in the LD fraction. In contrast, Sec61α was found only in the bottom fractions (fractions 6–8), in which most membrane and soluble proteins were contained. SEL1L was immunoprecipitated before SDS–PAGE for efficient detection. (B) Immunofluorescence microscopy confirmed that UBXD8, UBXD2, and p97 distributed in LDs. LDs and nuclei were labeled with BODIPY493/503 (green) and 4′,6-diamidino-2-phenylindole (blue), respectively. p97 distributed throughout the cytoplasm and nucleoplasm, but a conspicuous concentration around LDs was observed. Bars, 10 μm. (C) Double immunofluorescence labeling revealed that UBXD8 (red) and an ER luminal protein, PDI (green), were distributed on the opposite hemisphere of LDs. Bar, 5 μm. (D) Domain structure and hydropathy plot of UBXD8. A domain showing high hydrophobicity was named the HP domain. Full-length UBXD8 and mutants lacking one of the domains (ΔUBA, ΔHP, ΔUAS, or ΔUBX) were conjugated to the carboxy terminus of GFP and expressed in Huh7 cells. (E) GFP-UBXD8(ΔUBA), GFP-UBXD8(ΔUAS), GFP-UBXD8(ΔUBX), and GFP-HP showed concentration around LDs like GFP-UBXD8(FL), but GFP alone and GFP-UBXD8(ΔHP) distributed diffusely in the cytoplasm, indicating that the HP domain is necessary and sufficient for UBXD8 to localize in the LD. LDs were stained red by BODIPY558/568-C12. Bars, 10 μm.