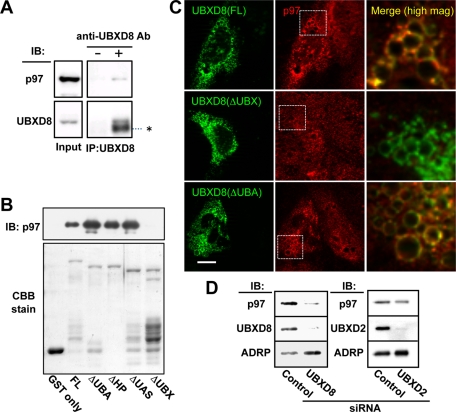
FIGURE 2:
UBXD8 recruited p97 to LDs by the UBX domain. (A) Immunoprecipitation of endogenous UBXD8 from Huh7 cell lysates caused cosedimentation of endogenous p97. The input lane was loaded with 2% of the cell lysate. The IgG heavy chain is indicated by an asterisk. (B) In vitro pull-down assay between recombinant p97 and recombinant GST-UBXD8 proteins. An equal amount of GST-free p97 was incubated with full-length and mutant GST-UBXD8 proteins, and the bound fraction was examined by Western blotting. All but GST and GST-UBXD8(ΔUBX) pulled down p97. The GST proteins in the input are shown by Coomassie brilliant blue staining. (C) Endogenous UBXD8 in Huh7 cells was knocked down by siRNA, and siRNA-resistant cDNA of GFP-tagged UBXD8(FL), UBXD8(ΔUBX), or UBXD8(ΔUBA) was transfected. In cells expressing the GFP-tagged proteins (green), LDs that harbored GFP-UBXD8(FL) and UBXD8(ΔUBA) showed an intense accumulation of endogenous p97 (red), but those with UBXD8(ΔUBX) did not. Merged pictures show a high magnification of the rectangular areas. Bar, 10 μm. (D) Huh7 cells were depleted of UBXD8 or UBXD2 by RNAi and the amount of p97 in the LD fraction was compared. p97 in the LD fraction was reduced significantly after UBXD8 knockdown but less so by UBXD2 knockdown. ADRP is shown as a loading control of LDs.