Abstract
Study Design
Retrospective study of male and female spinal osteoarthritis, characterized by lateral spine thoracolumbar radiographs, in humans and nonhuman primates
Objective
To characterize differences in prevalence and vertebral distribution of spinal osteoarthritis between men and women, between male and female macaques, and between the two phylogenetically related genera.
Summary of Background Data
Naturally occurring spinal osteoarthritis manifests similarly in humans and rhesus macaques. Other types of osteoarthritis particularly of the knee and hip have revealed gender differences in humans. In regard to spinal osteoarthritis, gender differences have been noted but without consistent results. Sex differences in macaques have not been examined.
Methods
Radiographic evidence of disc space narrowing and osteophytosis was assessed using an atlas scoring method. Prevalence was determined according to sex, age, body mass (for macaques only) and spinal location (human T4-L5; macaque T8-L7).
Results
Average scores in macaques differed between the sexes, but they did not differ between men and women. The pattern of involvement along the spine was the same in male and female monkeys but differed between men and women: women had more thoracic involvement and men had more lumbar involvement. Overall, monkeys had a significantly higher prevalence of osteoarthritis than humans.
Conclusion
The appearance of sex differences in the prevalence of osteoarthritis is most likely a proxy measure for the affect of body mass. Sex differences were apparent in monkeys due to the fact that males are significantly heavier than females. No gender difference in prevalence was apparent in humans and there is substantial overlap in body mass between men and women. Differences in the location of osteoarthritic involvement along the spine between men and women were obscured when only average scores were examined.
Keywords: Sex differences, spinal osteoarthritis, osteophytosis, disc space narrowing, primate comparative studies
Introduction
Spinal osteoarthritis (OA) is a significant health concern affecting millions of people and incurring millions of dollars in medical and disability costs1,2. While risk factors for spinal OA such as age, body mass, trauma, and genetic influences have been studied, the influence of gender differences remains unclear. Studies of gender differences in OA of the hand, hip and knee3–6 are more common due to available clinical and surgical data. Investigations relating to gender differences in spinal OA7–13 are fewer and have reported conflicting results in prevalence and in the patterns of involvement of the vertebral regions.
Primate models, particularly those using rhesus macaques (Macaca mulatta, Zimmermann 1780)14–19, have proven useful in the study of spinal OA, given that the condition manifests similarly in humans and nonhuman primates19–21. As with human spinal OA studies, the influence of sex differences has not been well characterized. The aim of this study was to determine whether or not sex differences are present in the occurrence and patterning of spinal OA by examining radiographic evidence in men and women and male and female macaques.
Materials and Methods
Monkeys
The sample consisted of 41 male (aged 13–30, average 21 years) and 27 female (aged 11–27, average 19 years) rhesus macaques (M. mulatta). Animals were housed at the Wisconsin National Primate Research Center (WNPRC) and have complete medical histories. Using these records, we calculated an average adult body mass (in kg) for each animal. Monkey ages were converted to human equivalent ages for the purpose of direct comparison to human data using 1:3.5 year human:rhesus macaque age ratio14–16. Animal care details were in accordance with Institutional Animal Care and Use Committee and are described in detail elsewhere22. These monkeys are part of an ongoing study of the effects of long-term dietary restriction on morbidity and mortality in primates22,23. The difference between control and dietary restriction animals was non-significant in our analyses and consequently, the two groups were combined and are discussed as a single group. The radiographs used in this analysis were randomly selected from a larger, longitudinal radiographic dataset in order to create a cross-sectional sample comparable to that available for our human subjects.
Humans
This group consisted of 173 men (aged 21–101, average 50 years) and 244 women (aged 20–82, average 51 years) randomly selected from individuals who had lateral thoracolumbar radiographs taken at a level 1 trauma center from January 1, 2000, to December 31, 2001. The radiographs were part of a standard protocol to rule-out spinal injury after a traumatic event. All study subjects were trauma-free. Institutional Review Board approval was obtained prior to examination of the human radiographs. Selection criteria for women are described elsewhere 24 and the same criteria applied to men in this study. Body mass was not available for people.
Radiographic Assessment
Lateral thoracolumbar radiographs were available for each subject. All monkey images (N=68) were obtained onsite at WNPRC and were assessed and scored by a single observer (AED), blind to the animal’s age, body mass and sex. Human radiographs (N=417) were obtained from the trauma center and were scored by a single observer (PAK) blind to the subject’s age. Scoring protocols were the same for all radiographs. Each vertebral level was scored for disc space narrowing (DSN), defined as a change in disc height relative to the adjacent intervertebral spaces, and osteophytosis (OST), defined by the presence of osteophytes on the anterior vertebral margins. An atlas scoring method was used: 0 for unaffected sites; 1, 2 or 3 for slight, moderate, or severe involvement, respectively, as has been applied previously17,25.
Radiographic clarity of the more cranial thoracic vertebrae varied considerably in all subjects. In monkeys, therefore, evaluation was restricted to spaces T8/T9 through L6/L7 for DSN and vertebral bodies T8 to L7 for OST. In humans, evaluation was restricted to spaces T4/T5 through L4/L5 for DSN and bodies T5-L5 for OST. In all cases, the lumbosacral joint was not scored for DSN due to excessive variability in height and shape of the disc 26,27. Variability in the clarity of some radiographs prohibited the scoring of some areas, resulting in variable sample sizes at individual sites. Monkey vertebral score counts ranged from 13 to 68. Human vertebral score counts ranged from 331 to 416.
Several cumulative scores were calculated. An overall average DSN and OST score was computed for each individual. Thoracic and lumbar regional scores as well as specific vertebral level scores were computed for different groups: all monkeys, all humans, and males and females by genera. No combined scores between DSN and OST were calculated.
Unilinear and multilinear regression analyses (STATA, College Station, TX) were performed with age, sex, and body mass as covariates for DSN and OST scores. Two multilinear models were examined in monkeys. The first excluded body mass measures to allow for direct comparison between the monkey and human data, while the second model included body mass. Differences between the sexes in average scores for vertebral levels were evaluated with Student’s T-test. Statistical significance was established at an alpha of p < 0.05. Analysis of pattern differences at individual vertebral levels in OST and DSN scores between sexes was performed using MATLAB® (MathWorks, Natick, MA).
Results
Monkeys
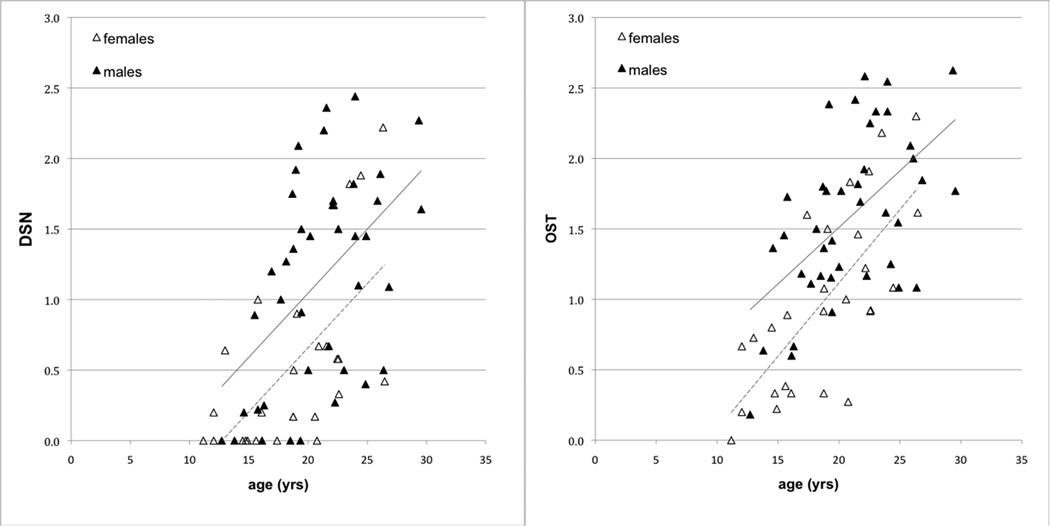
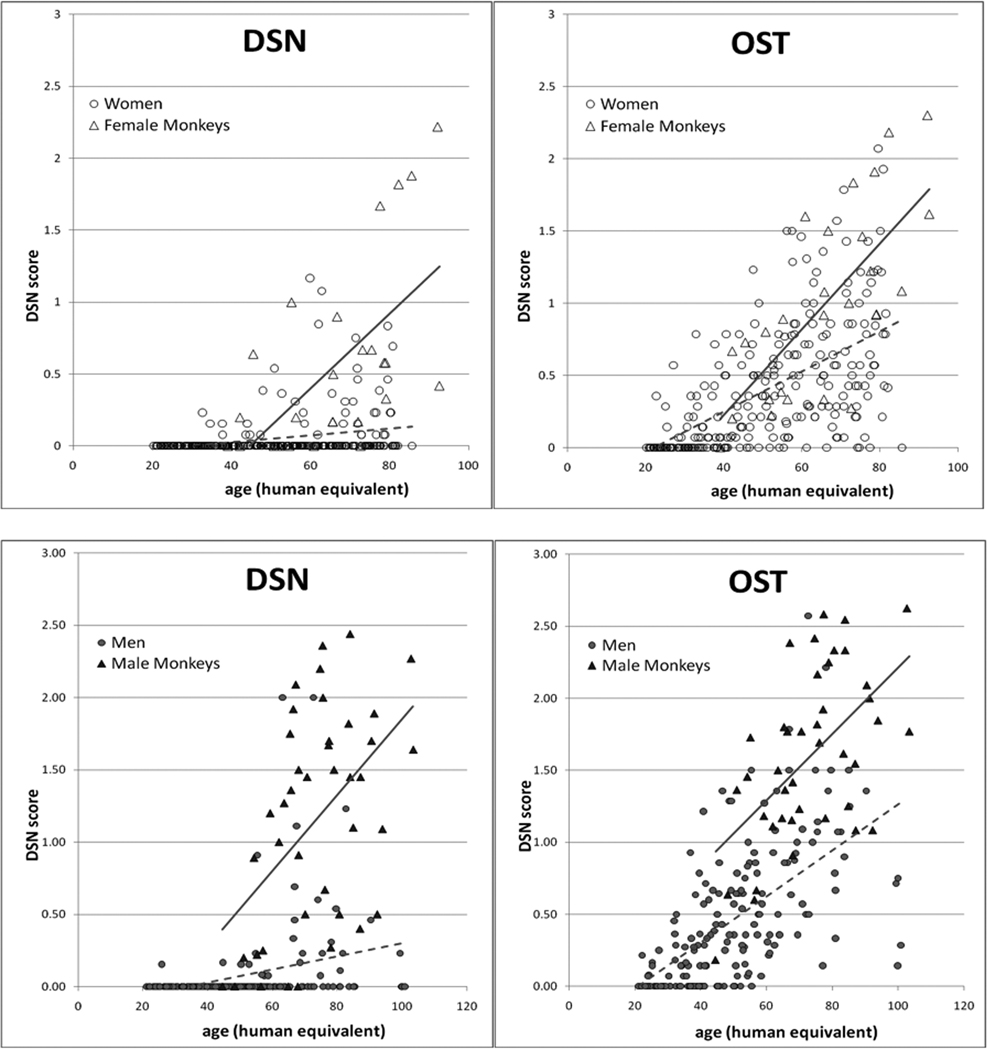
DSN and OST were present but variable throughout the sample, with some older individuals having low scores and some younger individuals having high scores (Figure 1). The youngest female with DSN was 12 years, the youngest male 14 years. For OST, the youngest individuals of both sexes were 12 years. In univariate analyses, age, sex and body mass were all significant predictors of OA (Table 1). Males had significantly higher average overall, thoracic, and lumbar scores than females.
Figure 1.
Average disc space narrowing and osteophytosis scores in female (dotted trend line) and male (solid trend line) monkeys.
Table 1.
Unilinear and multilinear regression results.
| Humans (N=417) |
Macaques (n=68) |
|||
|---|---|---|---|---|
| Dependent Variables | r2 | p | r2 | p |
|
DSN | ||||
| Univariate Analyses | ||||
| age | 0.072 | <0.000 | 0.331 | <0.000 |
| sex | 0.002 | <0.317 | 0.148 | <0.001 |
| body mass | na | na | 0.180 | <0.000 |
| Multivariate Analyses | ||||
| 1. full model | 0.075 | 0.394 | ||
| age | <0.000 | <0.000 | ||
| sex | <0.227 | <0.012 | ||
| 2. full model | na | 0.470 | ||
| age | na | <0.000 | ||
| sex | na | <0.812 | ||
| body mass | na | <0.000 | ||
|
OST | ||||
| Univariate Analyses | ||||
| age | 0.334 | <0.000 | 0.427 | <0.000 |
| sex | 0.005 | <0.173 | 0.197 | <0.000 |
| body mass | na | na | 0.255 | <0.000 |
| Multivariate Analyses | ||||
| 1. full model | 0.341 | 0.512 | ||
| age | <0.000 | <0.000 | ||
| sex | <0.035 | <0.001 | ||
| 2. full model | na | 0.625 | ||
| age | na | <0.000 | ||
| sex | na | <0.842 | ||
| body mass | na | <0.000 | ||
In a multilinear model of OA excluding body mass as a predictor, age and sex were significant and together explained 39% of the variability in DSN and 51% in OST. When body mass was included, sex was no longer a significant predictor in the model. Together age and body mass explained 47% of the variability in DSN and 62% in OST. Limiting the analysis to the thoracic or lumbar averages of DSN and OST revealed the same relationships.
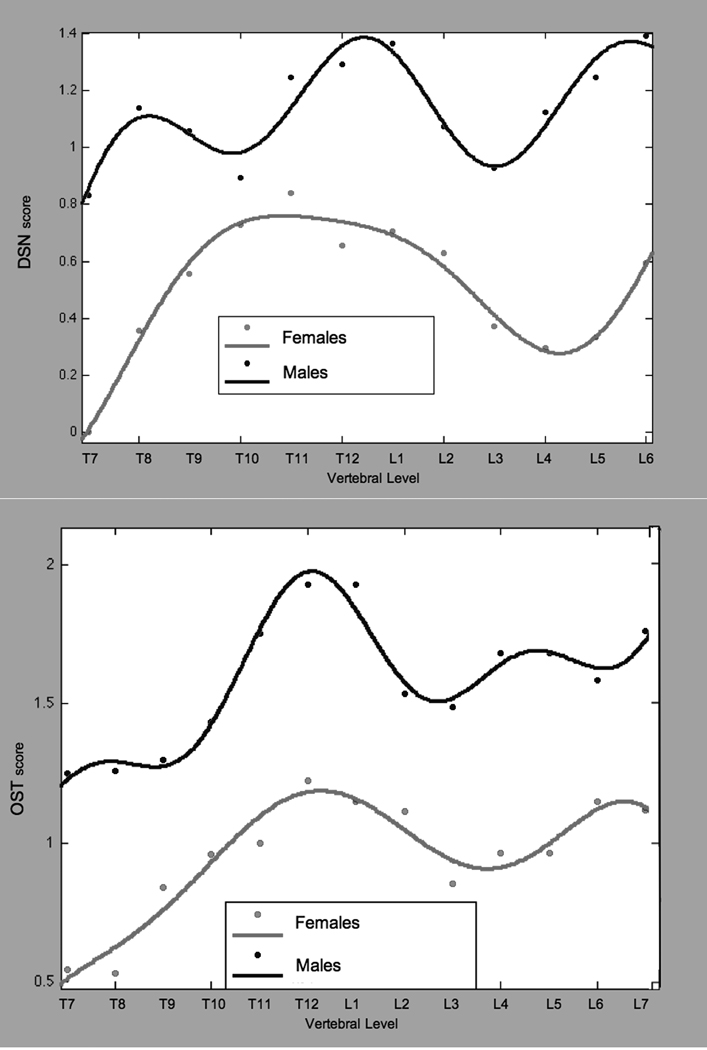
Sex differences were apparent in the pattern of DSN and OST across vertebral levels (Figure 3a). The DSN curve for males included two maxima, at T12/L1 and at L6/L7. In females, there was a broad maximum spanning the lower thoracic region (T10-L1) and another at L6/L7. Males had significantly higher DSN scores at every vertebral level (p < 0.045) except T9-T11. The OST curves were similar for both sexes with two maxima, at T12/L1 and L6/L7. Males had significantly higher OST scores at all vertebral levels (p < 0.037).
Figure 3.
a. Average disc space narrowing and osteophytosis in monkeys, by vertebral level.
b. Average disc space narrowing and osteophytosis in humans, by vertebral level.
Humans
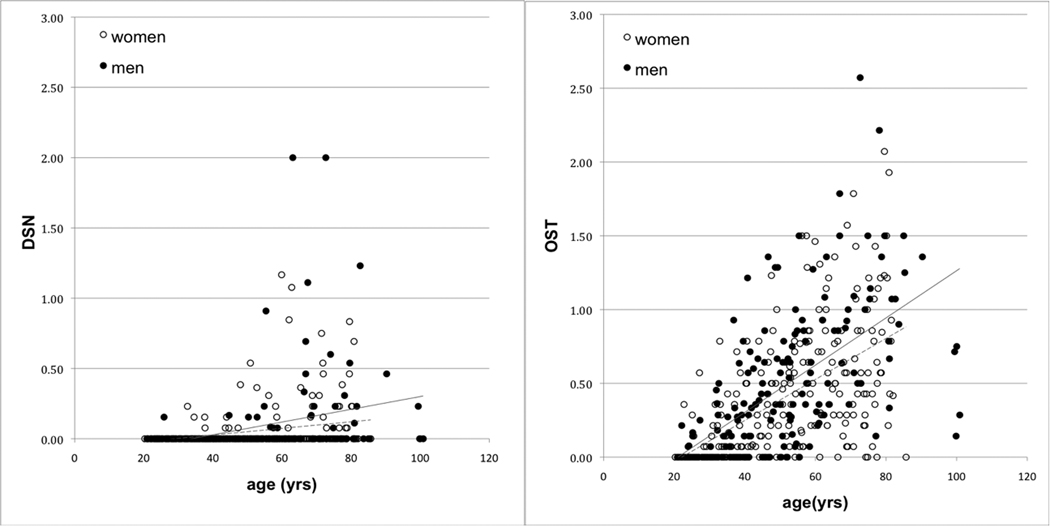
As with monkeys, DSN and OST were present throughout the human sample (Figure 2). The youngest woman with DSN was 32 years, the youngest man 25years. For OST, the youngest individuals of both sexes were 22 years. In univariate analysis, there was a significant positive association between age and OA outcome, but no gender difference was apparent (Table 1).
Figure 2.
Average disc space narrowing and osteophytosis scores in human women (dotted trend line) and men (solid trend line).
In the multilinear analysis of DSN gender was non-significant and age explained only 8% of the variability in the model. In the multilinear analysis of OST, age and gender together explained 34% of the variability, only 1% more than that explained by age alone. In regard to thoracic or lumbar OA, age was always a significant predictor (p<0.000) but gender was significant only for lumbar OST (p < 0.000).
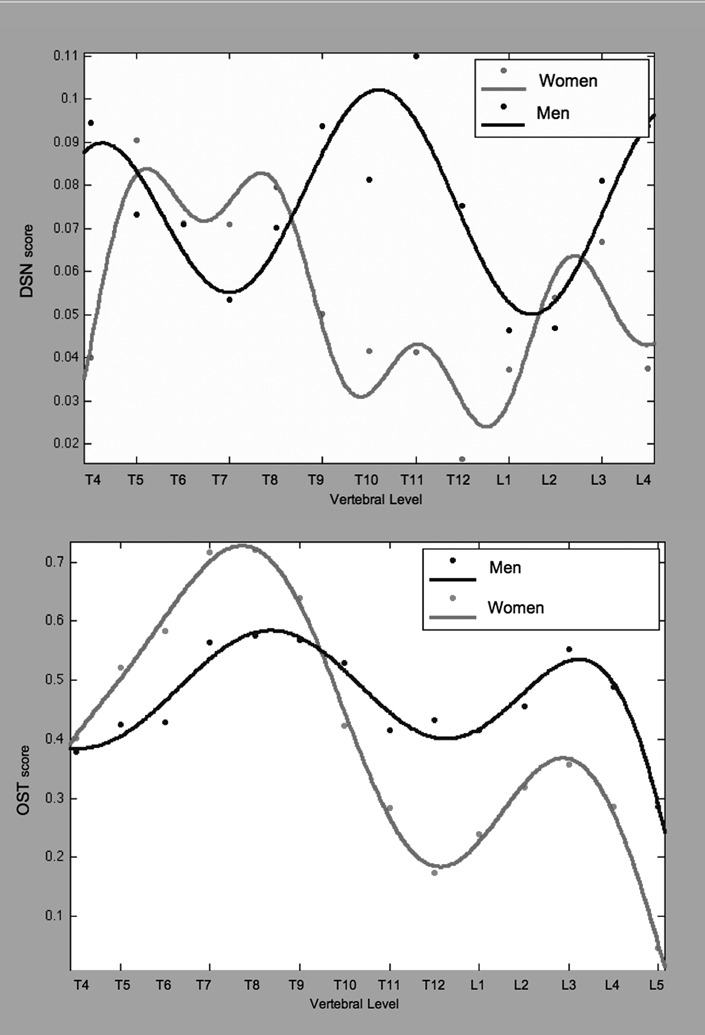
The pattern of OA across the spine was different in women than in men (Figure 3b). Men had higher DSN scores than women at T11/T12, and L4/L5 (p < 0.045). The shape of the DSN curves differed as well, where women had a minimum from T9 to L1 men exhibited a peak. Both genders showed a double maxima pattern in OST with peaks at similar locations, T7/T8 and L3/L4. Women had significantly higher scores, however, in the mid thoracic region (T6-T8, p < 0.049) while men had significantly higher scores in the thoracolumbar joint and lumbar regions (T11-L5, p < 0.048).
Inter-generic
After transforming monkey ages to human equivalents based on 1:3.5 year ratio 14–16, analysis of DSN and OST scores showed that age, sex, and species were significant predictors in unilinear (p<0.000) and multilinear regressions (p<0.001). Increasing age, maleness, and being a monkey were all positively associated with higher OA outcomes Figure 4). Together, age, sex and species explained 48% of the variation in average DSN scores and 55% of the variation in average OST scores.
Figure 4.
Average disc space narrowing and osteophytosis score comparisons between women (dotted trend line) and female monkeys (solid trend line) above, and men (dotted trend line) and male monkeys (solid trend line) below. Monkey ages have been adjusted to represent their human equivalent based on 1:3.5yr human:monkey age ratio 14–16.
The regions of greatest expression of OA were similar for males of both species with DSN and OST maxima located at the thoracolumbar and lumbosacral joints. Female monkeys and women were less similar: women showed more upper to mid-thoracic DSN and OST with significant minima at the thoracolumbar joint while female monkeys showed lower mid-thoracic DSN and OST and thoracolumbar maxima. Similarly in the lumbar region, women showed a mid lumbar maximum while female monkeys showed a minimum at the same location.
Discussion
Using demographic and radiographic data gathered from humans and rhesus macaques, we examined whether or not and, if so, how spinal OA differed between men and women, between male and female monkeys, and between the two genera. We found that average DSN and OST scores in monkeys differed between the sexes, until body mass was figured into the model and revealed that sex is likely a proxy measure for body mass. We found no sex difference in average scores in humans, likely due to the overlap in body mass in men and women but we did not have data to confirm this suspicion. The pattern of spinal involvement did not differ between male and female macaques; however, the pattern did differ between men and women. Our results revealed greater thoracic involvement in women and greater lumbar involvement in men, aspects that were obscured by average scores.
An advantage of this study is that we were able to use consistent methodology to evaluate OA in both groups providing us with easily comparable datasets. Our study is limited in a number of ways, including the relatively small number of macaques in our study group. The macaques in this study are, however, similar to those in other studies14–17 with larger sample sizes so it seems unlikely that their data are substantially skewed. Also, we lacked body mass data for the human subjects in this study, which made it impossible to tease apart the affect of gender from that of body mass in women and men. Lastly, because OA is so closely associated with growth and aging, which in primates follow a sigmoid curve over time, a linear regression model may not be the best fit. Using cross-sectional data, however, does not allow us to fit sigmoidal growth curves with any reliability, nor are we able to determine changes in growth rates that might affect the prevalence and severity of OA in humans and macaques. More extensive longitudinal analyses are warranted.
The relationships between body mass, body size, and sex are complex in primates. Sexual dimorphism in body size is a common trait in primates. In macaques, males are up to 60% larger than females28, but in humans, sexual dimorphism in body size is considerably less, with men being, on average, 10% larger than women29. In monkeys, sex was non-significant in our multivariate analysis when mass was included, which can be attributed to the strong correlation of sex with body mass in macaques. It is possible that the significant association of sex in the univariate analysis may have functioned as a partial proxy for body mass. Although we were not able to obtain body masses for the humans, it is likely that men and women overlapped to a large degree in mass, making sex a poor predictor of mass.
In many human studies that include body mass, measures are obtained through self-reporting, which can provide unreliable, often under-estimated, measures30. Reliable body mass can be measured on site at the time of radiography, but even this may not be a useful metric. In nonhuman primates particularly, but also in humans, single body mass measures can reflect periods of abnormality due to illness or other temporary influences and may be less indicative of an individual’s true average adult body mass and, hence, its biomechanical interactions with the musculoskeleton over time. Body mass averaged over an individual’s lifetime would appear to be a better measure of how body mass and OA may interact. A cross-sectional study of humans, therefore, can never provide a measure similar to the longitudinal mass records available for our macaque subjects. Consequently, a captive monkey model may give us the most insight.
Few studies have examined spinal OA in male and female macaques. DeRousseau16 found no sex bias in her examination of degenerative disc disease in M. mulatta, but noted that her sample size may have lacked the power to detect a sex difference. Colman and colleagues also looked at both male15 and female M. mulatta14, but their OA reporting differs between papers and applies only to lumbar spine prevalence. Lumbar OA in males and females was significantly and positively associated with age but the authors found no association with body mass in either study. The lack of association may be related to their use of mass at time of radiography rather than average adult body mass, as discussed above.
Gender differences in human spinal OA have been examined in several studies7–12,31, but researchers employed various definitions and methods of evaluation. For example, Lawrence7 focused on “disc degeneration” as a combination of several characteristics including osteophytosis, disc space narrowing, disc slippage and sclerosis. Miller and colleagues8 defined and graded “disc generation” according to macroscopic wear to the disc itself. Other authors have focused solely on osteophytosis, but have graded it on different scales. Nathan31 examined OST across the entire spine and found that the pattern of involvement differed between the sexes with men having higher frequency and higher severity of OST than women. O’Neill and colleagues9 examined OST across the thoracolumbar spine, also finding men had higher frequency and severity of OST than women, but that the patterns of vertebral involvement were similar in both sexes. These results are also similar to studies looking solely at the lumbar spine10,11. Snodgrass12 looked at the thoracolumbar spine but found a positive male bias only in regard to lumbar OST. Although we cannot compare our results directly with those of other studies due to the differences in methodology, our findings do not support the general trend that men have higher levels and more severe involvement of OA (especially OST) than women. Our findings of gender differences by spinal region do, however, support those of Lawrence7, who found women had more OST in the thoracic spine and men in the lumbar spine.
The intergeneric differences we found may be relevant to future studies of OA using the macaque model and well as to future human studies. Overall, macaques had significantly higher prevalence and severity of spinal OA compared to humans. Differences exist in the habitual orientation, curvature, and biomechanical stresses in the two spines. Macaques are anatomical quadrupeds, though they spend a substantial amount of time sitting with their spines in a vertical orientation. The human spine is habitually vertically oriented with sagittal curvature and inherent flexibility and shock absorption. It is possible that the architecture of the bipedal spine may function in a protective manner in regard to OA, explaining the lower levels of DSN and OST seen in our human study group. Further examination of vertebral bone composition, and that of osteophytes in particular, as well as possible structural and biomechanical differences in human and macaque vertebrae may help to explain intergeneric differences in spinal OA.
There are several hypotheses regarding the etiology of osteophytes as structural responses to biomechanical stresses26,31–33. Nathan31 describes osteophytes as a reinforcing structural responses to spinal stress, noting that osteophytic bone is compact bone, appearing to be stronger than bone of the normal vertebral body. If osteophytes are a bony response to loading and movement related stresses, it would follow that a gender difference in OST distribution would correspond to the spinal region where gender-related stress differences are most apparent. Our data showed that men have significantly higher OST in the lumbar region and women in the thoracic. Because the lumbar spine carries the majority of weight-related spinal forces, bearing the stress of the entire upper portion of the body, it has been hypothesized that this region would be more susceptible to weight-related stresses. Miller and colleagues8 proposed that men, based on the trunk extensor musculature as well as the size and area differences in vertebral bodies, exert maximally 1.28 times the compressive force per unit cross-sectional area than women. While standing, men exert the same or slightly less compressive stress to the lumbar spine, but maximal exertions load the male spine with up to 28% more compressive stress and 72% higher compression forces than in women. The finding that women have more OST in the thoracic region has not been examined in detail and no hypotheses as to why this should occur have been proposed as yet. Future comparative work focusing on the differences in flexibility, load balancing, and compressive forces in the spine may further our understanding of why women and men differ in the distribution of OA.
In conclusion, intergeneric differences revealed much higher prevalence and severity of OA in monkeys than in humans. In macaques, males had more prevalent and severe OA than females, but no differences were apparent in patterning across the vertebral column. In contrast, no gender effect was apparent in humans with regard to prevalence or severity of OA, but there was a significant difference in the affected areas of the spine: women had higher thoracic involvement and men higher lumbar. These apparent differences in the manifestation of spinal OA in men and women present an area to be explored in future work.
Key Points.
Sex differences in the regional distribution and prevalence of spinal osteoarthritis were apparent in both humans and macaques, but the pattern is not the same.
Macaque males have significantly higher prevalence than macaque females.
Differences in prevalence between men and women appear to be regional -- men have higher lumbar involvement and women have higher thoracic involvement.
Gender differences in humans may indicate that body mass distribution and related biomechanical stress differences pose different risk factors for men and women.
Acknowledgements
This work was supported by NIH grants U01 AG21379, P01 AG-11915 and P51 RR000167. This research was conducted in part at the WNPRC which received support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01.
IRB and IACUC approval were obtained for this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The manuscript submitted does not contain information about medical device(s)/drug(s). Federal funds were received to support this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Contributor Information
Andrea E. Duncan, Department of Anthropology, University of Washington, Box 353100, Seattle, WA 98195-3100, USA.
Ricki J. Colman, Wisconsin National Primate Research Center, University of Wisconsin, Madison, WI 53715, USA.
Patricia A. Kramer, Departments of Anthropology and of Orthopaedics and Sports Medicine, University of Washington, Box 353100, Seattle, WA 98195-3100, USA.
References
- 1.WHO. The Global Economic and Healthcare Burden of Musculoskeletal Disease: World Health Organization. 2001
- 2.Leigh JP, Seavey W, Leistikow B. Estimating the costs of job related arthritis. Journal of Rheumatology. 2001;28:1647–1654. [PubMed] [Google Scholar]
- 3.Franklin J, Ingvarsson T, Englund M, et al. Sex Differences in the Association between Body Mass Index and Total Hip or Knee Joint Replacement Resulting from Osteoarthritis. Annals of the Rheumatic Diseases. 2009;68:536–540. doi: 10.1136/ard.2007.086868. [DOI] [PubMed] [Google Scholar]
- 4.Maillefert JF, Gueguen A, Monreal M, et al. Sex Differences in Hip Osteoarthritis: Results of a Longitudinal Study in 508 Patients. Annals of the Rheumatic Diseases. 2003;62:931–934. doi: 10.1136/ard.62.10.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srikanth VK, Fryer JL, Zhai G, et al. A Meta-analysis of Sex Differences Prevalence, Incidence and Severity of Osteoarthritis. Osteoarthritis and Cartilege. 2005;13:769–781. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 6.O'Connor MI. Osteoarthritis of the Hip and Knee: Sex and Gender Differences. Othropedic Clinics of North America. 2006;37:559–568. doi: 10.1016/j.ocl.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence JS. Disc degeneration. Its frequency and relationship to symptoms. Annals of the Rheumatic Diseases. 1969;28:121–138. doi: 10.1136/ard.28.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller J, Schmatz C, Schultz A. Lumbar Disc Degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 1988;13:173–178. [PubMed] [Google Scholar]
- 9.O'Neill TW, McCloskey EV, Kanis JA, et al. The Distribution, Determinants, and Clinical Correlates of Vertebral Osteophytosis: A Population Based Survey. Journal of Rheumatology. 1999;26:842–848. [PubMed] [Google Scholar]
- 10.Pye SR, Reid DM, Smith R, et al. Radiographic features of lumbar disc degeneration and self-reported back pain. Journal of Rheumatology. 2004;31:753–758. [PubMed] [Google Scholar]
- 11.Shao Z, Rompe G, Schiltenwolf M. Radiographic Changes in the Lumbar Interveterbral Discs and Lumbar Vertebrae with Age. Spine. 2002;27:263–268. doi: 10.1097/00007632-200202010-00013. [DOI] [PubMed] [Google Scholar]
- 12.Snodgrass JJ. Sex Differences and Aging of the Vertebral Column. Journal of Forensic Science. 2004;49:458–463. [PubMed] [Google Scholar]
- 13.Yoshimura N, Dennison E, Wilman C, et al. Epidemiology of chronic Disc Degeneration and Osteoarthritis of the Lumbar Spine in Britain and Japan: A Comparative Study. Journal of Rheumatology. 2000;27:429–433. [PubMed] [Google Scholar]
- 14.Colman RJ, Kemnitz JW, Lane MA, et al. Skeletal effects of aging and menopausal status in female rhesus macaques. Journal of Clinical Endocrinology and Metabolism. 1999;84:4144–4148. doi: 10.1210/jcem.84.11.6151. [DOI] [PubMed] [Google Scholar]
- 15.Colman RJ, Lane MA, Binkley N, et al. Skeletal effects of aging in male rhesus monkeys. Bone. 1999;24:17–23. doi: 10.1016/s8756-3282(98)00147-1. [DOI] [PubMed] [Google Scholar]
- 16.Derousseau CJ. Aging in the Musculoskeletal System of Rhesus Monkeys: II. Degenerative Joint Disease. American Journal of Physical Anthropology. 1985;67:177–184. doi: 10.1002/ajpa.1330670303. [DOI] [PubMed] [Google Scholar]
- 17.Kramer PA, Newell-Morris LL, Simkin PA. Spinal degenerative disk disease (DDD) in female macaque monkeys: epidemiology and comparison with women. Journal of Orthopaedic Research. 2002;20:399–408. doi: 10.1016/S0736-0266(01)00122-X. [DOI] [PubMed] [Google Scholar]
- 18.Nuckley DJ, Kramer PA, Del Rosario A, et al. Intervertebral disc degeneration in a naturally occurring primate model: radiographic and biomechanical evidence. J Orthop Res. 2007;26:1283–1288. doi: 10.1002/jor.20526. [DOI] [PubMed] [Google Scholar]
- 19.Pritzker KP, Chateauvert J, Grynpas MD, et al. Rhesus Macaques As an Experimental Model for Degenerative Arthritis. Puerto Rico Health Sciences Journal. 1989;8:99–102. [PubMed] [Google Scholar]
- 20.Black A, Lane MA. Nonhuman primate models of skeletal and reproductive aging. Gerontology. 2002;48:72–80. doi: 10.1159/000048930. [DOI] [PubMed] [Google Scholar]
- 21.Platenburg R, Hubbard G, Ehler W, et al. Spontaneous disc degeneration in the baboon model: Magenetic resonance imaging and histopathologic correlation. Journal of Medical Primatology. 2001;30:268–272. doi: 10.1034/j.1600-0684.2001.d01-59.x. [DOI] [PubMed] [Google Scholar]
- 22.Colman RJ, Anderson RM, Johnson SC, et al. Caloric Restriction Delays Disease Onset and Mortality in Rhesus Monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramsey JJ, Colman RJ, Binkley NC, et al. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Experimental Gerontology. 2000;35:1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 24.Kramer PA. Prevalence and Distribution of Spinal Osteoarthritis in Women. Spine. 2006;31:2843–2848. doi: 10.1097/01.brs.0000245854.53001.4e. [DOI] [PubMed] [Google Scholar]
- 25.Lane NE, Nevitt MC, Genant HK, et al. Reliability of New Indices of Radiographic Osteoarthritis of the Hand and Hip and Lumbar Disc Degeneration. Journal of Rheumatology. 1993;20:1911–1918. [PubMed] [Google Scholar]
- 26.Quinnell RC, Stockdale HR. The significance of osteohpytes on lumbar vertebral bodies in relation to discographic findings. Clinical Radiology. 1982;33:197–203. doi: 10.1016/s0009-9260(82)80062-7. [DOI] [PubMed] [Google Scholar]
- 27.Cohn EL, Maurer EJ, Keats TE, et al. Plain Film Evaluation of Degenerative Disk Disease at the Lumbosacral Junction. Skeletal Radiology. 1997;26:161–166. doi: 10.1007/s002560050213. [DOI] [PubMed] [Google Scholar]
- 28.Leigh SR. Patterns of Variation in the Ontogeny of Primte Body Size Dimorphism. Journal of Human Evolution. 1992;23:27–50. [Google Scholar]
- 29.Rogers AR, Mukherjee A. Quantitative Genetics of Sexual Dimorphism in Human Body Size. Evolution. 1992;46:226–234. doi: 10.1111/j.1558-5646.1992.tb01997.x. [DOI] [PubMed] [Google Scholar]
- 30.Gorber SC, Tremblay M, Moher D, et al. A Comparison of Direct vs. Self-reported Measures for Assessing Height, Weight, and Body Mass Index: a Systematic Review. Obesity Reviews. 2007:8. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 31.Nathan H. Osteophytes of the verterbral column - an anatomical study of their development according to age, race, and sex with considerations as to their etiology and significance. Journal of Bone and Joint Surgery-American Volume. 1962;44:243–268. [Google Scholar]
- 32.Schmorl G, Junghanns H. The Human Spine in Health and Diseaseed. New York: Grune & Stratton, Inc; 1971. [Google Scholar]
- 33.Sokoloff L. The Biology of Degenerative Joint Diseaseed. Chicago: University of Chicago Press; 1969. [Google Scholar]