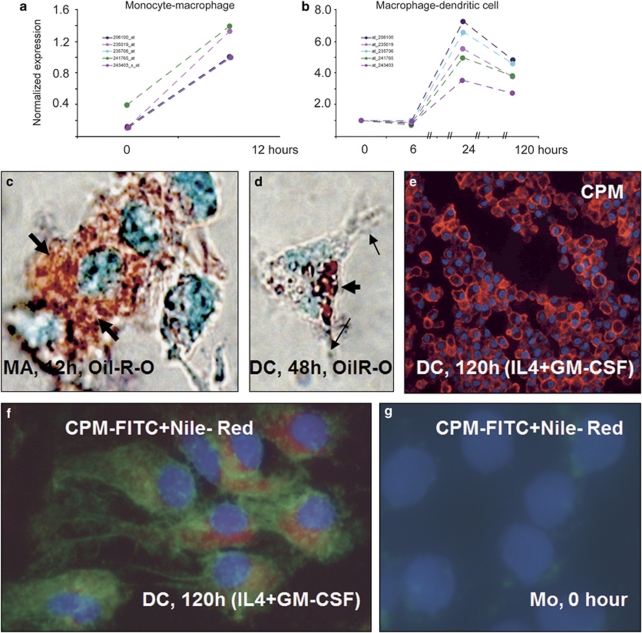
Figure 10.
Microarray data for CPM gene activation during monocyte-macrophage (MA) and MA-dendritic cell (DC) differentiations associated with serum lipids and cytokines. (a and b) Microarray analyses using various probe sets (identified by numbers with different color lines on graphs) show that during monocyte to MA differentiation, there is a robust acute CPM gene activation (a), which is increased further during monocyte-derived MA transition into early DCs induced by interleukin-4+granulocyte–macrophage-colony-stimulating factor (IL-4+GM-CSF) (b). (c and d) The lipid (Oil-Red-O) staining of these monolayer cells, viewed under partial phase-contrast lights, revealed remarkable lipid uptake by differentiating MAs (thick arrows of c), which appears to be correlated with CPM-gene upregulation. (d) At 48 h of culture with GM-CSF+IL-4, monocyte-derived differentiating cells having cytoplasmic projections (arrows), a typical dendritic cell morphology, still contain cytoplasmic lipids (thick arrow), albeit in lower quantity compared with cells of image c. (e) The section of paraffin-embedded cell pellets shows that at the end of differentiation time, CPM-protein expression remains elevated in immature DCs (red fluorescence), which reflects the gene-activating effects of the cytokines applied. (f) The combined fluorescent staining for CPM (green) and Nile-red for neutral lipids (red) simultaneously shows that the lipid content of DCs did not disappear completely by the end of the 5-day culturing time, while CPM-protein is still being expressed, consistent with the gene-expression profile of Figure 10b. (g) Monocytes contain neither lipid nor CPM, as expected. Images of (c and d) and (f, g) are from monolayer cells with × 63 original magnifications. Nuclear counterstaining: methyl-green for panels c and d and 4′-6-diamidino-2-phenylindole (DAPI) (blue fluorescence) for panels e, f and g.