Abstract
During the 20th century, the Hong Kong Chinese population experienced 2 abrupt but temporally distinct macroenvironmental changes: The transition from essentially preindustrial living conditions to a rapidly developing economy through mass migration in the late 1940s was followed by the emergence of an infant and childhood adiposity epidemic in the 1960s. The authors aimed to delineate the effects of these 2 aspects of economic development on mortality, thus providing a sentinel for other rapidly developing economies. Sex-specific Poisson models were used to estimate effects of age, calendar period, and birth cohort on Hong Kong adult mortality between 1976 and 2005. All-cause and cause-specific mortality, including mortality from ischemic heart disease (IHD), cardiovascular disease excluding IHD, lung cancer, other cancers, and respiratory disease, were considered. Male mortality from IHD and female mortality from other cancers increased with birth into a more economically developed environment. Cardiovascular disease mortality increased with birth after the start of the infant and childhood adiposity epidemic, particularly for men. Macroenvironmental changes associated with economic development had sex-specific effects over the life course, probably originating in early life. The full population health consequences of these changes are unlikely to manifest until persons who have spent their early lives in such environments reach an age at which they become vulnerable to chronic diseases.
Keywords: China, chronic disease, health transition, Hong Kong, mortality, myocardial ischemia, obesity, socioeconomic factors
With ongoing economic development and the epidemiologic transition, noncommunicable chronic diseases are expected to soon become the leading cause of mortality in developing countries as well as developed countries (1). However, in developing countries, the epidemiologic transition is taking place more rapidly than previously occurred in long-term developed countries, with a potentially different impact on population health (2). We used data on a population that recently went through a rapid epidemiologic transition to delineate the impact of such a transition on mortality, and thus to foretell what may happen in other populations currently undergoing rapid economic development.
The Chinese population of Hong Kong has a unique demographic history which fortuitously allows clear delineation of the inception of 2 abrupt, discrete macroenvironmental changes during the 20th century. The Hong Kong population was largely formed by mass migration in the mid-20th century (approximately 1945–1955) from essentially preindustrial China to relatively economically developed Hong Kong (3, 4), where rapid transition to a postindustrial economy occurred. As a result of this mass migration and rapid economic development, the population of Hong Kong experienced, first, in the late 1940s, a “step-change” in economic development, with corresponding improvements in nutrition, living conditions, and medical care, and second, in the 1960s, the inception of an epidemic of infant and childhood adiposity (5–7). Until the early 1960s, children in Hong Kong were generally thin; in 1963, approximately 1% (5) of Hong Kong children had a body mass index (weight (kg)/height (m)2) equivalent to an adult body mass index of 25 or higher, which is considered obese by Asian standards (8). By 1978, the body mass index distribution had shifted to the right (from a mean of 14.2 to 14.9 at age 5 years) (6), and by 1993, 13.8% of boys and 9.5% of girls were obese (body mass index equivalent to an adult body mass index of 25 or higher) at age 7 years (7). The distinct and well-defined timing of these 2 macroenvironmental changes in Hong Kong provided us with a unique opportunity to investigate separately the effects of rapid socioeconomic transition and childhood adiposity.
Adult lifestyle is a key driver of the changes in population health that occur with economic development. Nevertheless, living conditions throughout the life course and across generations may also be relevant, with early life being a potentially critical period for adult noncommunicable diseases (9). Uniquely in Hong Kong, the long-term effects of different levels of economic development during early life can be elucidated from a comparison of mortality by birth cohort, pre- and post-1945. The long-term effects of infant/child adiposity can be elucidated from a similar comparison of mortality pre- and post-1965. Conversely, population-wide effects, regardless of age at exposure, can be deduced from comparisons of mortality by calendar period. Here, we used age-period-cohort models to estimate the effects of age, calendar period, and birth cohort in Hong Kong on all-cause and cause-specific mortality for causes likely to change with the epidemiologic transition (cardiovascular disease (CVD), particularly heart disease, and cancer) (10) or with childhood adiposity (CVD and cancer) (11, 12). This allowed us to take advantage of Hong Kong's unique demographic history to identify the effects of some increasingly common macroenvironmental changes (rapid socioeconomic development and the epidemic of infant/child adiposity) on long-term population health. Moreover, Hong Kong experienced rapid economic development relatively early among developing economies, along with (as in many other Asian countries) relatively low rates of smoking among women (13). Hence, Hong Kong may act as a sentinel for other parts of the developing world currently undergoing similarly rapid economic development, such as mainland China.
MATERIALS AND METHODS
Data sources
We obtained age- and sex-specific data on midyear populations and all known deaths (i.e., registered deaths) by sex, year, and cause for 1976–2005 from the Hong Kong government Census and Statistics Department. Causes of death were coded using the Eighth Revision of the International Classification of Diseases for 1976–1978, the Ninth Revision for 1979–2000, and the Tenth Revision for 2001–2005. Most deaths in Hong Kong take place in hospitals (especially since 1970), facilitating accurate ascertainment of cause of death. Nevertheless, as in all developed countries, autopsy rates are falling and misdiagnoses are inevitable (14).
Outcomes
We considered as primary outcomes all-cause mortality and mortality from major causes expected to be associated with economic development—ischemic heart disease (IHD), other CVD excluding IHD, lung cancer, and other cancers excluding lung cancer. We considered lung cancer separately because changes in smoking patterns with economic development may underlie some of the changes in population health. No information on smoking was collected before 1983 in Hong Kong. Birth cohort trends in smoking may be inferred from birth cohort effects for lung cancer mortality, because lung cancer is mainly caused by smoking and is usually fatal. For completeness, we also considered the other major medical causes of death—respiratory diseases and all other medical causes (mainly digestive disorders; genitourinary diseases; infectious and parasitic diseases; and endocrine, nutritional, and metabolic diseases and immunity disorders). We also considered external causes (accidents, suicides, and homicides) as a “control” outcome to identify systematic changes.
Data analysis
Mortality rates were expressed per 100,000 population and were directly standardized to the World Standard Population (15). We used 11 5-year age groups ranging from 30–34 years to 80–84 years and 6 5-year calendar time periods ranging from 1976–1980 to 2001–2005, producing 16 birth cohorts ranging from 1894–1898 to 1969–1973.
We used sex-specific Poisson regression to estimate relative risks by age, period, and birth cohort, with 95% confidence intervals (specified in the Appendix). A fundamental problem inherent in age-period-cohort models is that the 3 components (i.e., cohort = period − age) are linearly dependent, making it impossible to estimate all 3 simultaneously in a regression model. There are several ways to overcome this nonidentifiability problem, including using an arbitrary additional reference constraint, estimating slopes (curvature) rather than regression coefficients, and fitting nonlinear effects for 1 or more components (16). The first method allows presentation of estimated effects as relative risks on age and time scales, although the nonidentifiability problem remains and interpretation should focus on second-order changes (i.e., changes in slopes or inflection points) rather than the absolute values of the estimated risks. The second method directly estimates the slopes (curvature) and is useful in identifying inflection points but has only recently come into use. The third method is appropriate only when there is biologic evidence of nonlinear effects (16).
We adopted the commonly used technique of an additional arbitrary reference constraint for the period effect (17–20), because initial analysis showed that the period effect did not vary as much as the age and cohort effects. In addition, 2 period effects may possibly be similar, whereas disease risk usually varies with age. We plotted the estimates for age, period, and cohort to facilitate the visual identification of second-order changes. To confirm our interpretations, we also plotted the estimated curvature components, which clarify when second-order changes occur. We chose the age group 55–59 years, the time periods 1981–1985 and 1996–2000, and the 1934–1938 birth cohort as reference categories. Results did not vary in terms of second-order changes with use of different reference categories for the period effects (see Web Figure 1, which is posted on the Journal’s Web site (http://aje.oxfordjournals.org/). We also assessed the contribution of age, period, and birth cohort by means of Akaike's Information Criterion; a lower value indicates a better-fitting model and hence a significant change in effect through time for the relevant component. All analyses were implemented in R software, version 2.5.0 (R Development Core Team, Vienna, Austria).
RESULTS
Age-standardized mortality rates
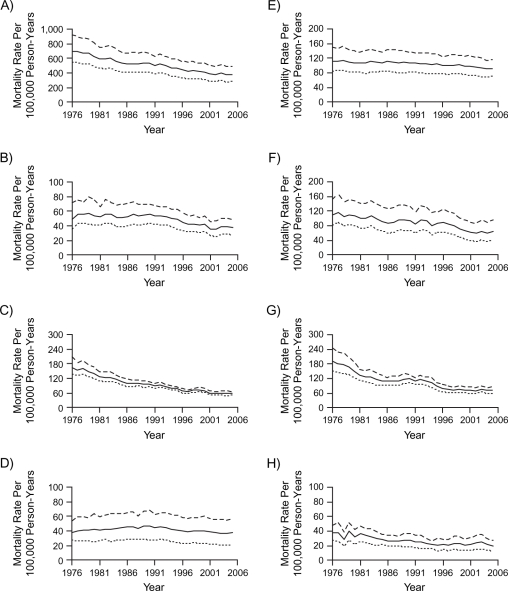
Figure 1 shows the observed age-standardized rates of all-cause and cause-specific mortality in Hong Kong from 1976 to 2005. There was a decline among both sexes, particularly for other CVD (excluding IHD), respiratory diseases, and all other medical causes, with less change for IHD, lung cancer, other cancers, and external causes.
Figure 1.
Age-standardized mortality rates in Hong Kong, 1976–2005, among men (dashed line), women (dotted line), and both sexes (solid line), for: A) all causes, B) ischemic heart disease, C) other cardiovascular diseases excluding ischemic heart disease, D) lung cancer, E) other cancers excluding lung cancer, F) respiratory disease, G) all other medical causes, and H) external causes.
Age, period, and cohort effects
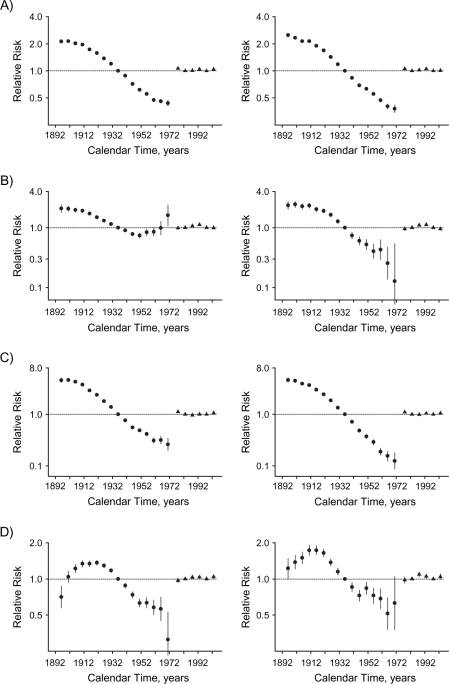
For almost all causes, age, period, and birth cohort contributed to the observed changes in mortality. Models including all 3 components fitted best (Table 1). The only exception was other cancers among women, where there was no period effect. Figure 2 shows the relative risks by period and birth cohort, from which second-order changes (inflection points) have been identified alongside examination of the curvature components (Figure 3). Web Figure 2 (http://aje.oxfordjournals.org/) shows the relative risks by age. In general, mortality increased with age and decreased with birth cohort. There was also a systematic downward inflection for men and women born around 1910. These birth cohorts mainly migrated from China in the late 1940s and had exceeded contemporary Chinese life expectancy at migration (21); with earlier birth cohorts, these represent increasingly strongly selected “healthy” migrants (22).
Table 1.
Akaike's Information Criterion Values for Age, Age-Period, Age-Cohort, and Age-Period-Cohort Models of the Effect of Socioeconomic Development on Risk of Mortality, Hong Kong, 1976–2005
| Mortality Outcome and Sex | Age Model | Age-Period Model | Age-Cohort Model | Age-Period Cohort Model |
| All causes | ||||
| Male | 20,154 | 2,087 | 1,138 | 939 |
| Female | 15,482 | 1,981 | 916 | 823 |
| Ischemic heart disease | ||||
| Male | 2,193 | 730 | 702 | 621 |
| Female | 2,023 | 796 | 628 | 538 |
| Other cardiovascular diseases excluding ischemic heart disease | ||||
| Male | 12,207 | 1,206 | 908 | 754 |
| Female | 9,912 | 1,470 | 731 | 661 |
| Lung cancer | ||||
| Male | 1,542 | 1,197 | 633 | 624 |
| Female | 1,235 | 921 | 578 | 560 |
| Other cancers excluding lung cancer | ||||
| Male | 2,855 | 1,826 | 728 | 717 |
| Female | 1,498 | 1,194 | 654 | 654 |
| Respiratory disease | ||||
| Male | 6,123 | 1,789 | 680 | 664 |
| Female | 5,111 | 1,233 | 639 | 574 |
| All other medical causes | ||||
| Male | 6,972 | 810 | 1,269 | 792 |
| Female | 4,408 | 930 | 884 | 679 |
| External causes | ||||
| Male | 1,151 | 754 | 713 | 646 |
| Female | 1,174 | 599 | 598 | 563 |
Figure 2.
Parameter estimates for cohort (circles) and period (triangles) effects of socioeconomic development on mortality in Hong Kong, 1976–2005, among men (left-hand panels) and women (right-hand panels), for: A) all causes, B) ischemic heart disease, C) other cardiovascular diseases excluding ischemic heart disease, D) lung cancer, E) other cancers excluding lung cancer, F) respiratory disease, G) all other medical causes, and H) external causes. Bars, 95% confidence interval.
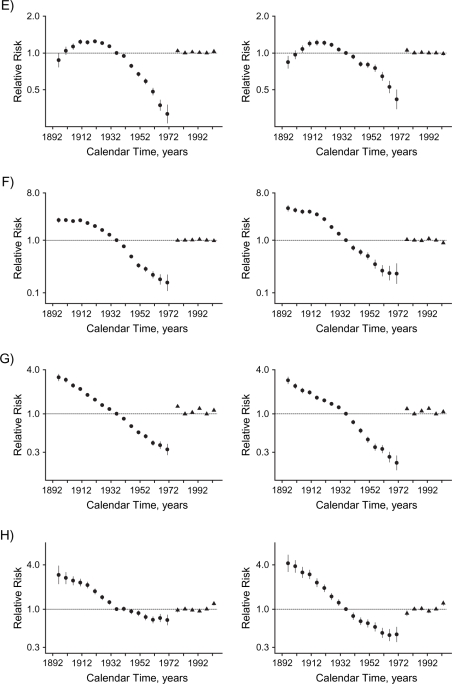
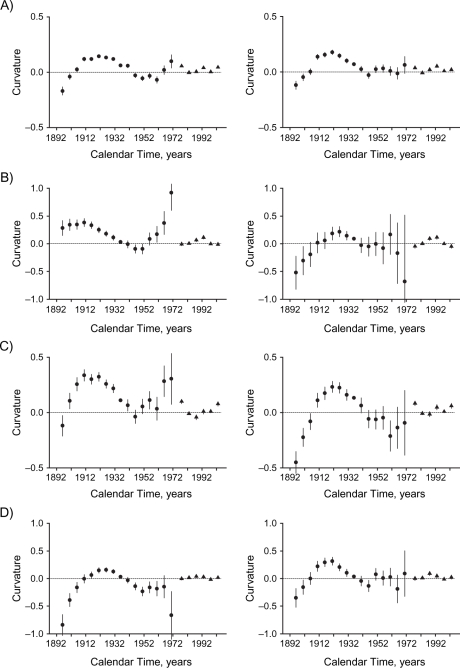
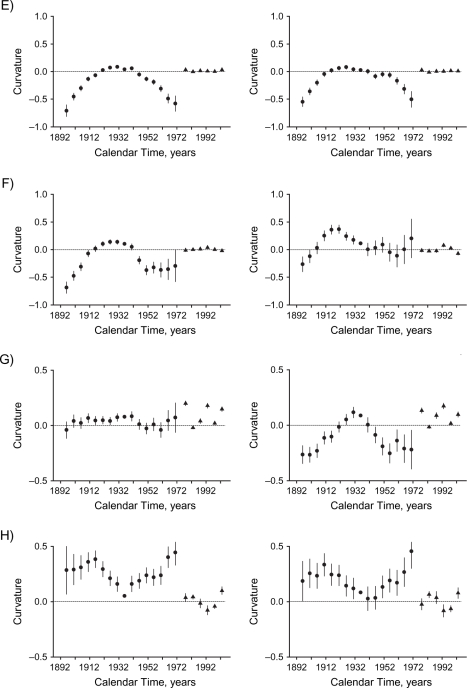
Figure 3.
Curvature components for cohort (circles) and period (triangles) effects of socioeconomic development on mortality in Hong Kong, 1976–2005, among men (left-hand panels) and women (right-hand panels), for: A) all causes, B) ischemic heart disease, C) other cardiovascular diseases excluding ischemic heart disease, D) lung cancer, E) other cancers excluding lung cancer, F) respiratory disease, G) all other medical causes, and H) external causes. Bars, 95% confidence interval.
All-cause mortality
All-cause mortality increased with age. There were upward inflections among women born in the 1940s, corresponding to the first generation of women born into a more developed environment, and then at the inception of the childhood adiposity epidemic for men and women born in the 1960s. These changes can be most clearly seen from the curvature components (Figure 3). For both sexes, the period effect had an upward inflection in the 1980s and a downward inflection in the 1990s.
Mortality from specific causes
IHD.
IHD mortality increased with age, although less steeply among older men (approximately ages ≥60 years). Changes by birth cohort differed between men and women. Among men, there was a marked turning point, such that the birth cohort effect reversed direction from decreasing to increasing, in the 1940s for the first generation of men born into a relatively developed environment. In contrast, there was no change by birth cohort in the 1940s among women. The trend in subsequent birth cohorts was not completely smooth. Wide confidence intervals indicate that any inflections could be chance fluctuations. There was an upward inflection in the period effect for men in the 1980s and a downward inflection for both sexes in the 1990s.
Other CVD excluding IHD.
Mortality from other CVD, excluding IHD, increased with age. The birth cohort curve for men had an upward inflection in the 1940s for the first generation of men born into a more developed environment. The birth cohort curve had an upward inflection in the 1960s, coincident with the inception of the childhood adiposity epidemic and most clearly seen from the curvature components (Figure 3); this was more evident in men than in women. The period effects in both sexes had upward inflections in the 1980s.
Lung cancer.
Lung cancer mortality increased with age, although less steeply at older ages. The birth cohort curve had a downward inflection for both sexes in the early 1920s and an upward inflection among women in the late 1940s but among men in the early 1950s—that is, after the inception of economic development. The trend in subsequent birth cohorts was not completely smooth in either sex. The wide confidence intervals indicate that any inflections could be chance fluctuations. The period effects in both sexes had downward inflections in the 1980s.
Other cancers excluding lung cancer.
Other cancer mortality increased with age. The birth cohort curve had a downward inflection for both sexes around the early 1920s, while there was an upward inflection for the first generation of women (but not men) born into a more developed environment in the late 1940s, followed by a downward inflection approximately 10 years later. Period effects were not obvious and did not contribute among women (Table 1).
Respiratory disease.
Respiratory mortality increased with age. The birth cohort curve had a downward inflection around the 1910s for women and slightly later for men. There was downward inflection in the early 1930s for men, followed by an upward inflection in the early 1950s—that is, distinct from the inception of economic development or childhood adiposity. Among women, there was upward inflection in the 1940s. There was an upward inflection in the period effect among women in the 1980s and downward inflections for both sexes in the 1990s.
All other medical causes.
Mortality from all other medical causes increased with age. The birth cohort curve had no marked inflection for men, while there was a downward inflection for women in the late 1930s, followed by a deceleration in the 1950s that was distinct from the inception of economic development and childhood adiposity.
External causes.
Mortality from external causes was consistent with age until around ages 70–74 years, when it started to increase. The birth cohort curve inflections did not clearly coincide with the inception of economic development (1940s) or the childhood adiposity epidemic (1960s). There was an upward inflection among men around the mid-1930s, followed by a downward inflection in the early 1950s and an upward inflection in the early 1960s for both sexes. The period effects in both sexes had an upward turning point in the 1990s.
DISCUSSION
Economic development and the correspondingly improved nutrition, living conditions, and medical care were generally associated with decreasing mortality. In addition, our findings illustrate the possible long-term effects of 2 distinct macroenvironmental events (rapid economic transition and the emerging epidemic of infant and child adiposity) on all-cause and cause-specific adult mortality by age, period, and birth cohort. Common, population-wide exposure to radically improved living conditions over a compressed time frame during the mid-20th century had long-term effects which differed by sex. With birth into a more developed environment, there appeared to be relative increases in cancer mortality for women and increases in CVD mortality, particularly IHD mortality, for men. In contrast, the emerging epidemic of infant and childhood adiposity had long-term effects which were similar by sex. People born after the start of the childhood adiposity epidemic in the 1960s appeared to have relatively higher mortality from all causes and from other CVD, which was most evident for men.
Economic transition
The increased IHD risk for men but not women, associated with birth into a more developed environment, is consistent with the hypothesis that the biologic pathway underlying increased IHD in men with economic development is environmentally and perhaps intergenerationally driven by higher levels of sex steroids during growth (23–25). Pubertal sex steroids have well-known physiologic effects that are detrimental with regard to central obesity (26, 27) and lipid profile (28–32) in men, with correspondingly opposite effects in women (26–29). Metabolic profile in adolescence tracks into adult life (33). Moreover, the increased risk of other cancers among women with the same exposure (i.e., birth into a more developed environment) is consistent with that predicted on the same theoretical basis, due to nutritionally driven levels of sex steroids during puberty (25). These effects appear to be specific to growing up in a developed environment, because once the proportion of the population who had grown up in Hong Kong stabilized at approximately 80% (in the 1960s birth cohorts) (17), some increases stopped. Conversely, these effects are unlikely to have been due to smoking, because the birth cohort curves for lung cancer did not mirror those for IHD, other CVD, or other cancers. These findings for IHD are also consistent with the sexually dimorphic secular trends in IHD seen historically with economic development in Western populations (34, 35). At a local level, the findings also explain why Hong Kong has not yet experienced an epidemic of IHD with contemporary economic development. Until very recently, most of the Hong Kong men who were of an age at which they were vulnerable to IHD had grown up in very limited conditions in China (3). On the other hand, our findings are apparently inconsistent with a previous study in Hong Kong (36); however, that study did not include recent birth cohorts or recent deaths and so could not detect changes by birth cohort in the 1940s. Our findings for cancer are consistent with an increase in hormonally driven (breast and ovarian) cancers, accompanying economic development or migration to more economically developed locations (37–40).
The lack of any downward inflection in CVD mortality with birth into a more developed environment (approximately the 1940s) is not consistent with Western studies, in which markers of early living conditions, such as leg length and height, are negatively associated with CVD (41–43). However, that association is less marked in non-Western populations (44–46) and is not congruent with the association between height and CVD risk across countries (41). As such, it suggests that the association between better childhood conditions and lower CVD risk in Western populations may be epidemiologically stage-specific or perhaps the result of residual confounding by intergenerational socioeconomic position (44) rather than due to childhood conditions. The lack of any clear downward inflection in mortality from other cancers with birth into a more developed environment may be arising from the specific pattern of cancers with different etiologies. Because of the rapid history of economic development, cancers with a long latency associated with infections, such as stomach cancer, nasopharyngeal cancer, and liver cancer, are still relatively common, although their incidence is declining (47), while cancers associated with more plentiful childhood conditions, such as colorectal, prostate, and breast cancer (48), are becoming more common (47). However, a study of cancer incidence in relation to socioeconomic development may be more illuminating. Alternatively, it is possible that the lack of any downturn in mortality from CVD or other cancers with birth into improved living conditions in Hong Kong is due to the self-selection of healthy migrants with, specifically, daughters prone to cancer and sons prone to IHD.
Mortality from external causes did not have a pattern similar to that of CVD, cancer, and respiratory disease. However, external causes were only a control outcome used to verify that the results for the other outcomes were not merely reflections of systematic changes.
Childhood adiposity epidemic
With the emergence of the childhood adiposity epidemic in the 1960s, the upward inflections by birth cohort for CVD are consistent with the effect of adiposity on CVD risks (49). This could indicate that infancy and childhood are critical periods when adiposity particularly affects risk or that the cumulative impact of lifetime adiposity starting in childhood drives risk. Childhood adiposity is positively associated with heart disease in adulthood (50). There is no record of when adult adiposity emerged in Hong Kong. However, there is no obvious effect of increasing adult adiposity, perhaps because adult adiposity had already reached a plateau by the start of the period considered in 1976. Alternatively, there may have been 2 opposing changes since 1976 of improved treatment for CVD and increased adiposity.
These distinct effects of economic transition and childhood adiposity are potentially relevant to many developing countries, where economic transition may be even more compressed, with an almost immediate transition from preindustrial conditions to adiposity in young and old alike. Under these circumstances, the full population health consequences of economic transition and the epidemic of infant and childhood adiposity on mortality may not be evident until persons who have spent their early lives in such macroenvironments reach an age at which they become vulnerable to noncommunicable chronic diseases—probably 50 or 60 years after the original changes took place. However, deleterious consequences of childhood adiposity can occur early in life, as evidenced by the rising rates of type 2 diabetes (51), high blood pressure (11), heart disease (11, 50), and other disorders that used to be medical oddities among children and adolescents. As such, our findings draw attention to the importance of taking action now during the current demographic window of opportunity.
Limitations
Despite these potential insights, there are caveats to these findings. First, our study was descriptive; we can only speculate about the etiologies of the observed changes. Second, our study considered only all-cause mortality and some broad categories of death. However, our aim in this study was to investigate the relative effects by age, period, and cohort of the epidemiologic transition from communicable diseases to noncommunicable diseases and the emerging epidemic of childhood adiposity. Third, our results depended on the quality of the mortality data, as do all other studies of the same type. During the study period, virtually all deaths in Hong Kong occurred in hospitals, facilitating accurate ascertainment of causes of death. Last, with overlapping confidence intervals at the turning points, especially towards the more recent birth cohorts, it is apparent that a longer period of data collection would help clarify the effects of the obesogenic environment that became more obvious in the 1970s, since not everyone has reached the age at which they will become vulnerable to the relevant chronic diseases. Because of this imprecision and the limitations of ecologic analyses of aggregate data, we are cautious in our interpretation. Particularly, women have lower death rates than men and wider confidence intervals for some causes of death in the recent cohorts, so the effect of the epidemic of childhood adiposity on CVD mortality in women is less clear than in men. Further work to quantify impacts on mortality into the future would be valuable; nevertheless, because CVD mortality is relatively common, small changes may have substantive effects.
Macroenvironmental changes brought on by economic transition increased mortality from IHD for men born into a more developed environment and possibly also from cancer for such women. The emergence of the childhood adiposity epidemic appeared to be associated with a relative increase in CVD mortality for both sexes, particularly for men, by birth cohort. The macroenvironmental changes associated with economic development have specific effects which extend over the life course, probably originating with early-life exposures. The full effects on mortality may not be evident until possibly 50 or 60 years after the original macroenvironmental changes took place. However, detrimental effects on CVD morbidity may occur in younger people and may be evident relatively quickly—hence the importance of taking action now.
Supplementary Material
Acknowledgments
Author affiliation: Department of Community Medicine, School of Public Health, Li Ka Shing Faculty of Medicine, University of Hong Kong, Hong Kong, People's Republic of China (Roger Y. Chung, C. Mary Schooling, Benjamin J. Cowling, Gabriel M. Leung).
This work received no financial support, except for a research postgraduate studentship to R. Y. C. from the University of Hong Kong.
The authors thank I. O. L. Wong for helpful guidance and assistance with data analysis.
Conflict of interest: none declared.
Glossary
Abbreviations
- CVD
cardiovascular disease
- IHD
ischemic heart disease
APPENDIX
We used sex-specific Poisson regression to estimate relative risks (with 95% confidence intervals) by age, period, and birth cohort. We assumed that the number of observed mortality cases, cij, out of a population of nij women in age group i in time period j, followed a Poisson distribution with mean μij. Under the full age-period-cohort model, the mean is specified as
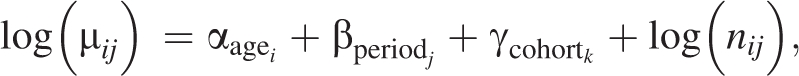 |
where αagei (i = 1,…, I, I = 11) is the age effect, βperiodj is the period effect (j =1,… J, J = 6), γcohortk is the cohort effect (k = 1,…, K, K = 16, and k = I +j −i), and log(nij) is the offset term.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030 [electronic article] PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yusuf S, Reddy S, Ounpuu S, et al. Global burden of cardiovascular diseases. Part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104(22):2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 3.Maddison A. Paris, France: Organisation for Economic Co-operation and Development; 2001. The World Economy: A Millennial Perspective. (OECD Development Centre Studies) [Google Scholar]
- 4.Vaughan TD, Dwyer DJ. Some aspects of postwar population growth in Hong Kong. Econ Geogr. 1966;42:37–51. [Google Scholar]
- 5.Chang KSF, Lee MMC, Low WD, et al. Standards of height and weight of Southern Chinese children. Far East Med J. 1965;1(3):101–109. [Google Scholar]
- 6.Family Health Service and Statistical Unit, Medical and Health Department, Government of Hong Kong. Survey on weight, height and head circumference of pre-school children in April 1978. Soc Community Med Hong Kong Bull. 1980;11:45–52. [Google Scholar]
- 7.So HK, Nelson EA, Li AM, et al. Secular changes in height, weight and body mass index in Hong Kong children [electronic article] BMC Public Health. 2008;8:320. doi: 10.1186/1471-2458-8-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization; International Association for the Study of Obesity. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Sydney, New South Wales, Australia: Health Communications; 2000. International Obesity Task Force. [Google Scholar]
- 9.Kuh D, Ben-Shlomo Y. A Life Course Approach to Chronic Disease Epidemiology: Tracing the Origins of Ill-Health From Early to Adult Life. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2004. [Google Scholar]
- 10.Omran AR. The epidemiologic transition. A theory of the epidemiology of population change. Milbank Mem Fund Q. 1971;49(4):509–538. [PubMed] [Google Scholar]
- 11.Freedman DS, Dietz WH, Srinivasan SR, et al. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics. 1999;103(6):1175–1182. doi: 10.1542/peds.103.6.1175. [DOI] [PubMed] [Google Scholar]
- 12.Must A, Jacques PF, Dallal GE, et al. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med. 1992;327(19):1350–1355. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 13.Ho SY, Lam TH, Fielding R, et al. Smoking and perceived health in Hong Kong Chinese. Soc Sci Med. 2003;57(9):1761–1770. doi: 10.1016/s0277-9536(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 14.Tse GM, Lee JC. A 12-month review of autopsies performed at a university-affiliated teaching hospital in Hong Kong. Hong Kong Med J. 2000;6(2):190–194. [PubMed] [Google Scholar]
- 15.Ahmad OB, Boschi-Pinto C, Lopez AD, et al. Age Standardization of Rates: A New WHO Standard. (GPE Discussion Paper no. 31) Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 16.Holford TR. Understanding the effects of age, period, and cohort on incidence and mortality rates. Annu Rev Public Health. 1991;12:425–457. doi: 10.1146/annurev.pu.12.050191.002233. [DOI] [PubMed] [Google Scholar]
- 17.Wu P, Cowling BJ, Schooling CM, et al. Age-period-cohort analysis of tuberculosis notifications in Hong Kong from 1961 to 2005. Thorax. 2008;63(4):312–316. doi: 10.1136/thx.2007.082354. [DOI] [PubMed] [Google Scholar]
- 18.Wong IO, Cowling BJ, Schooling CM, et al. Age-period-cohort projections of breast cancer incidence in a rapidly transitioning Chinese population. Int J Cancer. 2007;121(7):1556–1563. doi: 10.1002/ijc.22731. [DOI] [PubMed] [Google Scholar]
- 19.Tarone RE, Chu KC. Evaluation of birth cohort patterns in population disease rates. Am J Epidemiol. 1996;143(1):85–91. doi: 10.1093/oxfordjournals.aje.a008661. [DOI] [PubMed] [Google Scholar]
- 20.Shahpar C, Li G. Homicide mortality in the United States, 1935–1994: age, period, and cohort effects. Am J Epidemiol. 1999;150(11):1213–1222. doi: 10.1093/oxfordjournals.aje.a009948. [DOI] [PubMed] [Google Scholar]
- 21.Zhao W, Chen J. Implications from and for food cultures for cardiovascular disease: diet, nutrition and cardiovascular diseases in China. Asia Pac J Clin Nutr. 2001;10(2):146–152. doi: 10.1111/j.1440-6047.2001.00224.x. [DOI] [PubMed] [Google Scholar]
- 22.Frisbie WP, Cho Y, Hummer RA. Immigration and the health of Asian and Pacific Islander adults in the United States. Am J Epidemiol. 2001;153(4):372–380. doi: 10.1093/aje/153.4.372. [DOI] [PubMed] [Google Scholar]
- 23.Schooling CM, Jiang CQ, Lam TH, et al. Life-course origins of social inequalities in metabolic risk in the population of a developing country. Am J Epidemiol. 2008;167(4):419–428. doi: 10.1093/aje/kwm329. [DOI] [PubMed] [Google Scholar]
- 24.Schooling CM, Leung GM. A socio-biological explanation for social disparities in non-communicable chronic diseases—the product of history? J Epidemiol Community Health. doi: 10.1136/jech.2008.086553. In press. [DOI] [PubMed] [Google Scholar]
- 25.Schooling CM, Lam TH, Ho SY, et al. Does economic development contribute to sex differences in ischaemic heart disease mortality? Hong Kong as a natural experiment using a case-control study [electronic article] BMC Public Health. 2008;8:32. doi: 10.1186/1471-2458-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen W, Punyanitya M, Silva AM, et al. Sexual dimorphism of adipose tissue distribution across the lifespan: a cross-sectional whole-body magnetic resonance imaging study [electronic article] Nutr Metab (Lond) 2009;6:17. doi: 10.1186/1743-7075-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brufani C, Tozzi A, Fintini D, et al. Sexual dimorphism of body composition and insulin sensitivity across pubertal development in obese Caucasian subjects. Eur J Endocrinol. 2009;160(5):769–775. doi: 10.1530/EJE-08-0878. [DOI] [PubMed] [Google Scholar]
- 28.Jaross W, Baehrecke M, Trübsbach A, et al. Effects of sexual maturation on serum lipoproteins. Endokrinologie. 1981;78(1):28–34. [PubMed] [Google Scholar]
- 29.Berenson GS, Srinivasan SR, Cresanta JL, et al. Dynamic changes of serum lipoproteins in children during adolescence and sexual maturation. Am J Epidemiol. 1981;113(2):157–170. doi: 10.1093/oxfordjournals.aje.a113080. [DOI] [PubMed] [Google Scholar]
- 30.Kirkland RT, Keenan BS, Probstfield JL, et al. Decrease in plasma high-density lipoprotein cholesterol levels at puberty in boys with delayed adolescence. Correlation with plasma testosterone levels. JAMA. 1987;257(4):502–507. [PubMed] [Google Scholar]
- 31.Roemmich JN, Rogol AD. Hormonal changes during puberty and their relationship to fat distribution. Am J Hum Biol. 1999;11(2):209–224. doi: 10.1002/(SICI)1520-6300(1999)11:2<209::AID-AJHB9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 32.Morrison JA, Barton BA, Biro FM, et al. Sex hormones and the changes in adolescent male lipids: longitudinal studies in a biracial cohort. J Pediatr. 2003;142(6):637–642. doi: 10.1067/mpd.2003.246. [DOI] [PubMed] [Google Scholar]
- 33.Ovesen L. Adolescence: a critical period for long-term tracking of risk for coronary heart disease? Ann Nutr Metab. 2006;50(4):317–324. doi: 10.1159/000094294. [DOI] [PubMed] [Google Scholar]
- 34.Nikiforov SV, Mamaev VB. The development of sex differences in cardiovascular disease mortality: a historical perspective. Am J Public Health. 1998;88(9):1348–1353. doi: 10.2105/ajph.88.9.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawlor DA, Ebrahim S, Davey Smith G. Sex matters: secular and geographical trends in sex differences in coronary heart disease mortality. BMJ. 2001;323(7312):541–545. doi: 10.1136/bmj.323.7312.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu IT, Li W, Wong TW. Effects of age, period and cohort on acute myocardial infarction mortality in Hong Kong. Int J Cardiol. 2004;97(1):63–68. doi: 10.1016/j.ijcard.2003.07.032. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu H, Ross RK, Bernstein L, et al. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991;63(6):963–966. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbone F, Filiberti R, Franceschi S, et al. Socioeconomic status, migration and the risk of breast cancer in Italy. Int J Epidemiol. 1996;25(3):479–487. doi: 10.1093/ije/25.3.479. [DOI] [PubMed] [Google Scholar]
- 39.Deapen D, Liu L, Perkins C, et al. Rapidly rising breast cancer incidence rates among Asian-American women. Int J Cancer. 2002;99(5):747–750. doi: 10.1002/ijc.10415. [DOI] [PubMed] [Google Scholar]
- 40.Kliewer EV, Smith KR. Ovarian cancer mortality among immigrants in Australia and Canada. Cancer Epidemiol Biomarkers Prev. 1995;4(5):453–458. [PubMed] [Google Scholar]
- 41.McCarron P, Okasha M, McEwen J, et al. McCarron et al. respond to “height-cardiovascular disease relation”: are all risk factors equal? Am J Epidemiol. 2002;155(8):690–691. doi: 10.1093/aje/155.8.690. [DOI] [PubMed] [Google Scholar]
- 42.Smith GD, Greenwood R, Gunnell D, et al. Leg length, insulin resistance, and coronary heart disease risk: the Caerphilly Study. J Epidemiol Community Health. 2001;55(12):867–872. doi: 10.1136/jech.55.12.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawlor DA, Taylor M, Davey Smith G, et al. Associations of components of adult height with coronary heart disease in postmenopausal women: the British Women's Heart and Health Study. Heart. 2004;90(7):745–749. doi: 10.1136/hrt.2003.019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schooling CM, Jiang C, Lam TH, et al. Height, its components, and cardiovascular risk among older Chinese: a cross-sectional analysis of the Guangzhou Biobank Cohort Study. Am J Public Health. 2007;97(10):1834–1841. doi: 10.2105/AJPH.2006.088096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langenberg C, Araneta MR, Bergstrom J, et al. Diabetes and coronary heart disease in Filipino-American women: role of growth and life-course socioeconomic factors. Diabetes Care. 2007;30(3):535–541. doi: 10.2337/dc06-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song YM, Smith GD, Sung J. Adult height and cause-specific mortality: a large prospective study of South Korean men. Am J Epidemiol. 2003;158(5):479–485. doi: 10.1093/aje/kwg173. [DOI] [PubMed] [Google Scholar]
- 47.Hong Kong Hospital Authority. Hong Kong Cancer Registry [database] Hong Kong, China: Hong Kong Hospital Authority; 2008. ( http://www3.ha.org.hk/cancereg/e_stat.asp). (Accessed December 2, 2008) [Google Scholar]
- 48.Gunnell D, Okasha M, Smith GD, et al. Height, leg length, and cancer risk: a systematic review. Epidemiol Rev. 2001;23(2):313–342. doi: 10.1093/oxfordjournals.epirev.a000809. [DOI] [PubMed] [Google Scholar]
- 49.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368(9536):666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 50.Baker JL, Olsen LW, Sørensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357(23):2329–2337. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinhas-Hamiel O, Dolan LM, Daniels SR, et al. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr. 1996;128(5):608–615. doi: 10.1016/s0022-3476(96)80124-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.