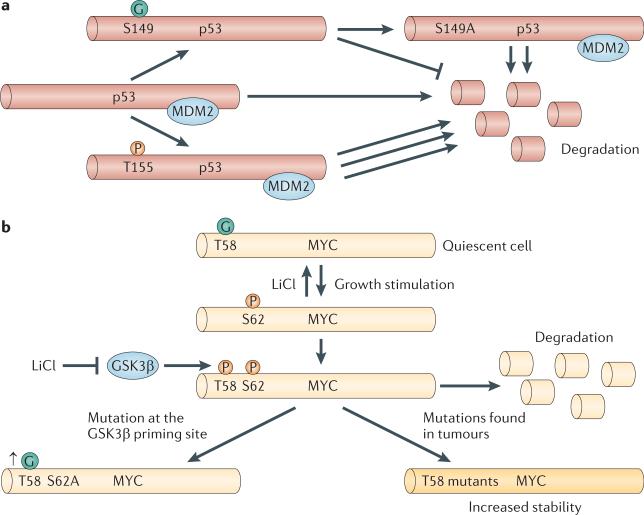
Figure 3. Regulation of transcription factors by O-GlcNAc.
a | The tumour suppressor p53 associates with the protein MDM2, which keeps p53 at low levels in cells by promoting its degradation. Phosphorylation (P) at Thr155 stimulates the rapid degradation of p53, and O-GlcNAcylation (the covalent attachment of β-D-N-acetylglucosamine (GlcNAc) sugars to serine or threonine residues of nuclear and cytoplasmic proteins; G) at Ser149 disrupts MDM2 binding and promotes p53 stability. Mutations abolishing O-GlcNAc on p53 enhances degradation. b | In quiescent cells, the oncoprotein MYC is O-GlcNAcylated at Thr58. Upon growth signals, the O-GlcNAc modification is rapidly removed, and the protein is phosphorylated at Ser62. This site is a priming site for glycogen synthase kinase 3β (GSK3β), which can then phosphorylate Thr58, leading to the rapid degradation of MYC. Numerous solid tumours have mutations at Thr58, which promotes increased MYC stability and altered gene transcription. Inhibition of GSK3β by lithium chloride (LiCl) or mutations at Ser62 increases O-GlcNAcylation at Thr58.