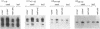Abstract
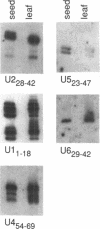
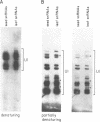
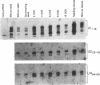
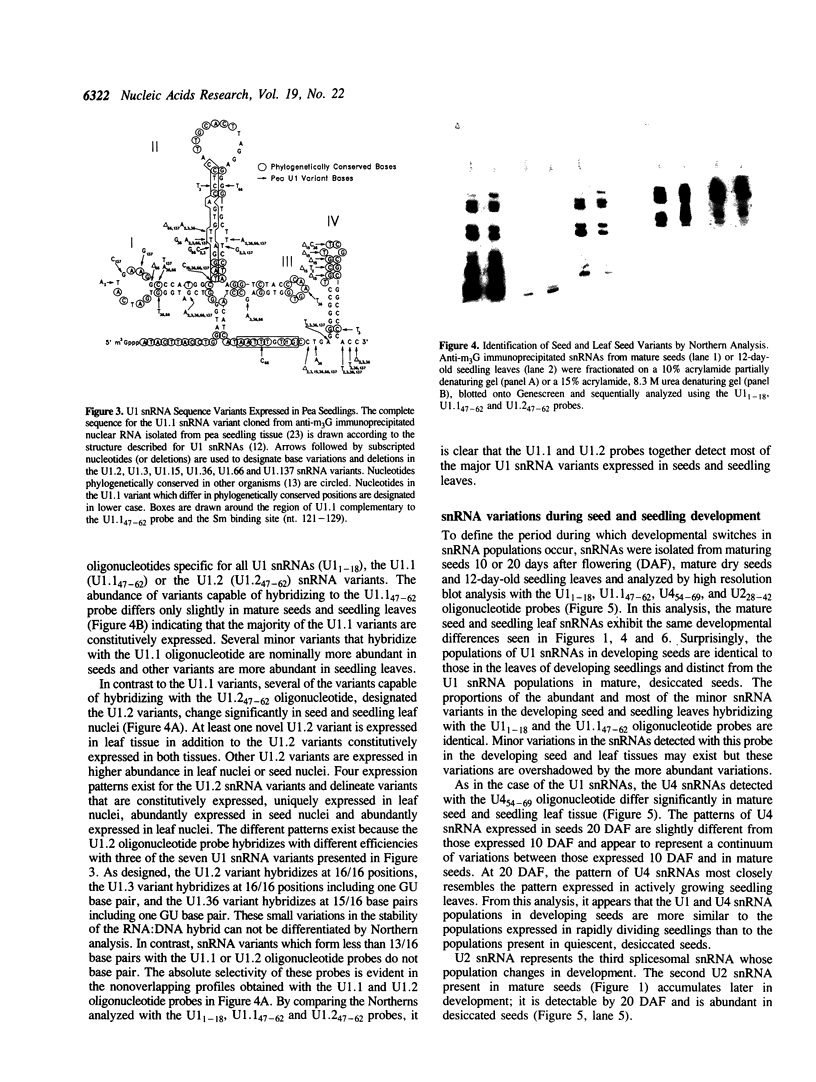
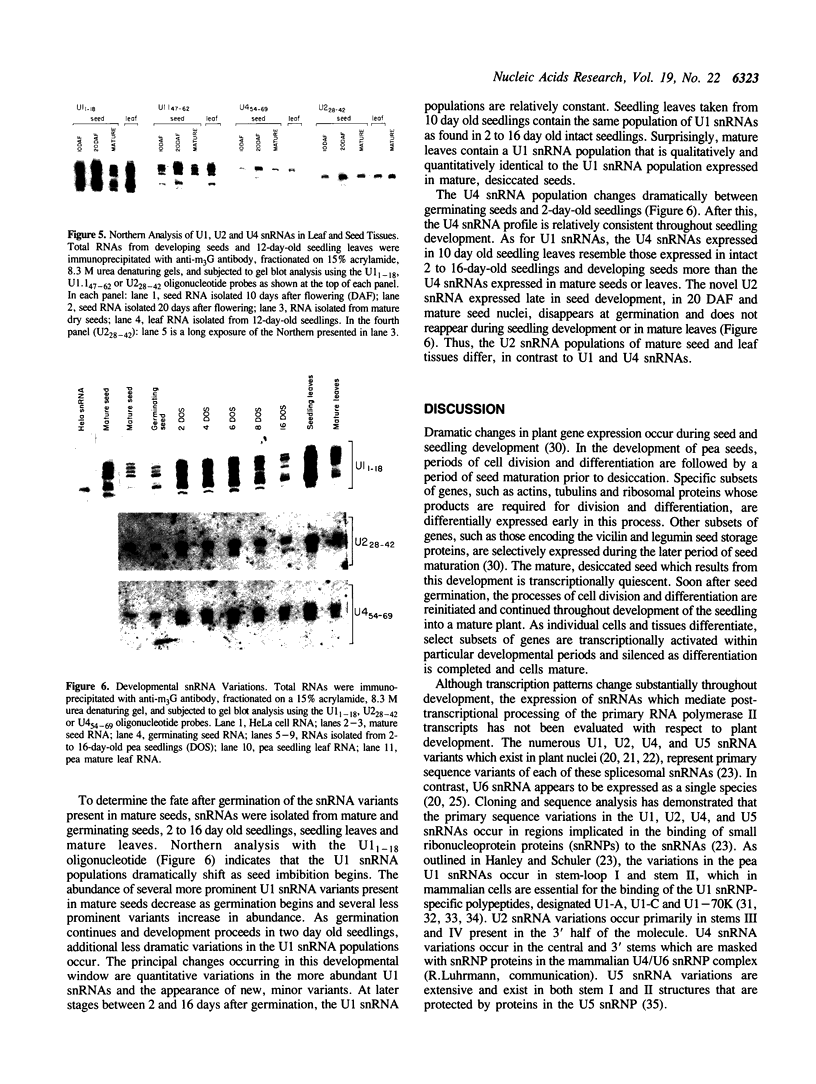
Although the number of plant U1, U2, U4 and U5 small nuclear RNA (snRNA) variants sequenced has steadily increased over the past few years, the function of these variants in plant splicing is still not understood. In an effort to elucidate the function of plant snRNA variants, we have examined the expression of U1-U6 snRNA variants during pea seedling development. In contrast to mammalian nuclei which express a single, abundant form of each snRNA, pea nuclei express several equally abundant variants of the same snRNA. Comparison of the snRNAs in pea seeds and seedlings has revealed that four (U1, U2, U4, U5) of the five snRNAs required for pre-mRNA splicing have differentially- and developmentally-regulated forms detectable on Northerns. Only U6 snRNA, which fractionates as a single species on Northerns, appears to be constitutively expressed. Switches in the expression of the pea U1, U2 and U4 snRNAs occur at three distinct stages in development: seed maturation, seed germination and seedling maturation. Surprisingly, the snRNA profiles of mature desiccated seeds and mature leaf tissues are nearly identical and different from developing seeds and seedlings suggesting that switches in the snRNA population occur at transitions between active and inactive transcription. Sequence analysis and differential hybridization of the U1 snRNA variants has demonstrated that some of the developmentally-regulated forms represent sequence variants. We conclude that select subsets of pea snRNAs accumulate at particular stages during plant development.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berget S. M., Robberson B. L. U1, U2, and U4/U6 small nuclear ribonucleoproteins are required for in vitro splicing but not polyadenylation. Cell. 1986 Aug 29;46(5):691–696. doi: 10.1016/0092-8674(86)90344-2. [DOI] [PubMed] [Google Scholar]
- Black D. L., Chabot B., Steitz J. A. U2 as well as U1 small nuclear ribonucleoproteins are involved in premessenger RNA splicing. Cell. 1985 Oct;42(3):737–750. doi: 10.1016/0092-8674(85)90270-3. [DOI] [PubMed] [Google Scholar]
- Black D. L., Pinto A. L. U5 small nuclear ribonucleoprotein: RNA structure analysis and ATP-dependent interaction with U4/U6. Mol Cell Biol. 1989 Aug;9(8):3350–3359. doi: 10.1128/mcb.9.8.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D. L., Steitz J. A. Pre-mRNA splicing in vitro requires intact U4/U6 small nuclear ribonucleoprotein. Cell. 1986 Aug 29;46(5):697–704. doi: 10.1016/0092-8674(86)90345-4. [DOI] [PubMed] [Google Scholar]
- Bringmann P., Appel B., Rinke J., Reuter R., Theissen H., Lührmann R. Evidence for the existence of snRNAs U4 and U6 in a single ribonucleoprotein complex and for their association by intermolecular base pairing. EMBO J. 1984 Jun;3(6):1357–1363. doi: 10.1002/j.1460-2075.1984.tb01977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brow D. A., Guthrie C. Spliceosomal RNA U6 is remarkably conserved from yeast to mammals. Nature. 1988 Jul 21;334(6179):213–218. doi: 10.1038/334213a0. [DOI] [PubMed] [Google Scholar]
- Chabot B., Black D. L., LeMaster D. M., Steitz J. A. The 3' splice site of pre-messenger RNA is recognized by a small nuclear ribonucleoprotein. Science. 1985 Dec 20;230(4732):1344–1349. doi: 10.1126/science.2933810. [DOI] [PubMed] [Google Scholar]
- Chandler P. M., Spencer D., Randall P. J., Higgins T. J. Influence of Sulfur Nutrition on Developmental Patterns of Some Major Pea Seed Proteins and Their mRNAs. Plant Physiol. 1984 Jul;75(3):651–657. doi: 10.1104/pp.75.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeland D. B., Sturtevant A. P., Schuler M. A. Molecular analysis of dicot and monocot small nuclear RNA populations. Plant Cell. 1989 Jun;1(6):633–643. doi: 10.1105/tpc.1.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes D. J., Kirschner M. W., Caput D., Dahlberg J. E., Lund E. Differential expression of multiple U1 small nuclear RNAs in oocytes and embryos of Xenopus laevis. Cell. 1984 Oct;38(3):681–689. doi: 10.1016/0092-8674(84)90263-0. [DOI] [PubMed] [Google Scholar]
- Goldberg R. B., Barker S. J., Perez-Grau L. Regulation of gene expression during plant embryogenesis. Cell. 1989 Jan 27;56(2):149–160. doi: 10.1016/0092-8674(89)90888-x. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Patterson B. Spliceosomal snRNAs. Annu Rev Genet. 1988;22:387–419. doi: 10.1146/annurev.ge.22.120188.002131. [DOI] [PubMed] [Google Scholar]
- Hamm J., Dathan N. A., Scherly D., Mattaj I. W. Multiple domains of U1 snRNA, including U1 specific protein binding sites, are required for splicing. EMBO J. 1990 Apr;9(4):1237–1244. doi: 10.1002/j.1460-2075.1990.tb08231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm J., van Santen V. L., Spritz R. A., Mattaj I. W. Loop I of U1 small nuclear RNA is the only essential RNA sequence for binding of specific U1 small nuclear ribonucleoprotein particle proteins. Mol Cell Biol. 1988 Nov;8(11):4787–4791. doi: 10.1128/mcb.8.11.4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley B. A., Schuler M. A. cDNA cloning of U1, U2, U4 and U5 snRNA families expressed in pea nuclei. Nucleic Acids Res. 1991 Apr 25;19(8):1861–1869. doi: 10.1093/nar/19.8.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto C., Steitz J. A. U4 and U6 RNAs coexist in a single small nuclear ribonucleoprotein particle. Nucleic Acids Res. 1984 Apr 11;12(7):3283–3293. doi: 10.1093/nar/12.7.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Harada F. New U1 RNA species found in Friend SFFV (spleen focus forming virus)-transformed mouse cells. J Biol Chem. 1985 Jun 25;260(12):7775–7782. [PubMed] [Google Scholar]
- Kiss T., Antal M., Solymosy F. Plant small nuclear RNAs. II. U6 RNA and a 4.5SI-like RNA are present in plant nuclei. Nucleic Acids Res. 1987 Jan 26;15(2):543–560. doi: 10.1093/nar/15.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T., Jakab G., Antal M., Pálfi Z., Hegyi H., Kis M., Solymosy F. Plant small nuclear RNAs. V. U4 RNA is present in broad bean plants in the form of sequence variants and is base-paired with U6 RNA. Nucleic Acids Res. 1988 Jun 24;16(12):5407–5426. doi: 10.1093/nar/16.12.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf G. M., Botros I. W., Stumph W. E. Developmental and tissue-specific expression of U4 small nuclear RNA genes. Mol Cell Biol. 1988 Dec;8(12):5566–5569. doi: 10.1128/mcb.8.12.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol A., Ebel J. P., Rinke J., Luhrmann R. U1, U2 and U5 small nuclear RNAs are found in plants cells. Complete nucleotide sequence of the U5 RNA family from pea nuclei. Nucleic Acids Res. 1983 Dec 20;11(24):8583–8594. doi: 10.1093/nar/11.24.8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. Differential accumulation of U1 and U4 small nuclear RNAs during Xenopus development. Genes Dev. 1987 Mar;1(1):39–46. doi: 10.1101/gad.1.1.39. [DOI] [PubMed] [Google Scholar]
- Lund E. Heterogeneity of human U1 snRNAs. Nucleic Acids Res. 1988 Jul 11;16(13):5813–5826. doi: 10.1093/nar/16.13.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Kahan B., Dahlberg J. E. Differential control of U1 small nuclear RNA expression during mouse development. Science. 1985 Sep 20;229(4719):1271–1274. doi: 10.1126/science.2412294. [DOI] [PubMed] [Google Scholar]
- Porter G., Brennwald P., Wise J. A. U1 small nuclear RNA from Schizosaccharomyces pombe has unique and conserved features and is encoded by an essential single-copy gene. Mol Cell Biol. 1990 Jun;10(6):2874–2881. doi: 10.1128/mcb.10.6.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Query C. C., Bentley R. C., Keene J. D. A specific 31-nucleotide domain of U1 RNA directly interacts with the 70K small nuclear ribonucleoprotein component. Mol Cell Biol. 1989 Nov;9(11):4872–4881. doi: 10.1128/mcb.9.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherly D., Boelens W., van Venrooij W. J., Dathan N. A., Hamm J., Mattaj I. W. Identification of the RNA binding segment of human U1 A protein and definition of its binding site on U1 snRNA. EMBO J. 1989 Dec 20;8(13):4163–4170. doi: 10.1002/j.1460-2075.1989.tb08601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollervey D. High level of complexity of small nuclear RNAs in fungi and plants. J Mol Biol. 1987 Jul 20;196(2):355–361. doi: 10.1016/0022-2836(87)90696-6. [DOI] [PubMed] [Google Scholar]
- Vankan P., Filipowicz W. Structure of U2 snRNA genes of Arabidopsis thaliana and their expression in electroporated plant protoplasts. EMBO J. 1988 Mar;7(3):791–799. doi: 10.1002/j.1460-2075.1988.tb02877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y., Weiner A. M. A compensatory base change in U1 snRNA suppresses a 5' splice site mutation. Cell. 1986 Sep 12;46(6):827–835. doi: 10.1016/0092-8674(86)90064-4. [DOI] [PubMed] [Google Scholar]
- Zhuang Y., Weiner A. M. A compensatory base change in human U2 snRNA can suppress a branch site mutation. Genes Dev. 1989 Oct;3(10):1545–1552. doi: 10.1101/gad.3.10.1545. [DOI] [PubMed] [Google Scholar]