Abstract
Somatic mutations in the additional sex comb-like 1 (ASXL1) gene have been described in various types of myeloid malignancies, including acute myeloid leukemia. Analysis of novel markers, such as ASXL1 mutations, in independent clinical trials is indispensable before considering them for clinical decision-making. We analyzed 882 well-characterized acute myeloid leukemia cases to determine the prevalence and prognostic impact of ASXL1 exon12 mutations. Truncating ASXL1 mutations were present in 46 cases (5.3%). ASXL1 mutations were inversely associated with FLT3 internal tandem duplications and mutually exclusive with NPM1 mutations. ASXL1 mutations were an unfavorable prognostic factor as regards survival (median overall survival 15.9 months vs. 22.3 months; P=0.019), with a significantly lower complete response rate (61% vs. 79.6%; P=0.004). In multivariate analyses, ASXL1 mutations were independently associated with inferior poor overall survival (HR 1.52, P=0.032). In conclusion, ASXL1 mutations are common mutations in acute myeloid leukemia and indicate a poor therapy outcome.
Keywords: acute myeloid leukemia, ASXL1 mutations, prognosis
Introduction
The Polycomb Group (PcG) and trithorax Group (trxG) proteins are epigenetic regulators.1 PcG proteins are required to epigenetically silence their target genes, whereas trxG proteins maintain transcriptional activation.2 Additional sex comb-like 1 (ASXL1) belongs to the Enhancer of trithorax and Polycomb genes, which are responsible for maintaining activation and silencing of PcG and trxG proteins.2 ASXL1 is a highly conserved gene consisting of an N-terminal ASX homology domain and a C-terminal plant homeodomain finger region (PHD).3 Somatic mutations in the ASXL1 gene have been described in various types of myeloid malignances: 11% myelodysplastic syndrome (MDS),4 8% myeloproliferative neoplasia,5 49% of chronic myelomonocytic leukemia,6 10.8% of de novo acute myeloid leukemia (AML)7 and 23% of post-MDS AML.8 Mutations were all found in exon 12 of the gene and cause truncation of the protein with loss of the PHD domain.4–8
The prevalence and prognostic value of ASXL1 mutations (ASXL1mut) in AML have not been extensively described.7 However, thorough analyses of novel markers in independent clinical trials are warranted before implementation of these markers in treatment decision-making can be considered. In this study, we determined the ASXL1 mutations in a cohort of 882 cases of AML. We investigated their distribution in relationship with cytogenetic and molecular risk categories, and we evaluated the impact of these mutations on survival and relapse.
Design and Methods
Bone marrow aspirates or peripheral blood samples of cohorts of patients with various hematologic malignancies were collected after obtaining written informed consent in accordance with the Declaration of Helsinki. This research has been reviewed and approved by the Erasmus University Medical Center Medical Ethical Review Committee (MEC-2004-030). AML patients were treated according to the HOVON (Dutch-Belgian Hematology-Oncology Cooperative group) protocols HO04, HO04A, HO29, HO42, HO42A and HO43 (http://www.hovon.nl).9–12
Mutation detection in FLT3 (internal tandem duplications (ITD) or tyrosine kinase domain mutations (TKD)), NPM1, N-RAS, IDH1 and IDH2, as well as EVI1 overexpression were performed as described previously.13–17
ASXL1 mutations in AML were determined by cDNA amplifications using Fw-ASXL1-Ex12 5′-CCACCCTGGGTGGTTAAAG-3′ and Rev-ASXL1-Ex12 5′-TCGCTGTAGATCTGACGTAC-3′. By this approach 83% of all known exon 12 ASXL1 mutations are identified (range P575 to R687 of ASXL1).7
All PCR reactions were carried out at an annealing temperature of 60°C in the presence of 25mM dNTP, 15 pmol primers, 2mM MgCl2, Taq polymerase and 1 x buffer (Invitrogen Life Technologies, Breda, The Netherlands). Cycling conditions were as follows: 1 cycle 5′ at 94°C, 35 cycles 1′ at 94°C, 1′ at 60°C, 1′ at 72°C, and 1 cycle 7′ at 72°C. All ASXL1 RT-PCR products were subjected to denaturing high performance liquid chromatography (dHPLC) analyses using a Transgenomics (Omaha, NE, USA) WAVE system. Samples were run at 64.1°C. PCR products showing aberrant dHPLC profiles were purified using the Multiscreen-PCR 96-well system (Millipore, Bedford, MA, USA) followed by direct sequencing with the appropriate forward and reversed primers using an ABI-PRISM3100 genetic analyzer (Applied Biosystems, Foster City, CA, USA). PCR products were sequenced with Fw-ASXL1-Ex12 and Rev-ASXL1-Ex12. We validated this strategy using 272 cases of AML that had been previously analyzed using PCR on genomic DNA followed by direct sequencing.
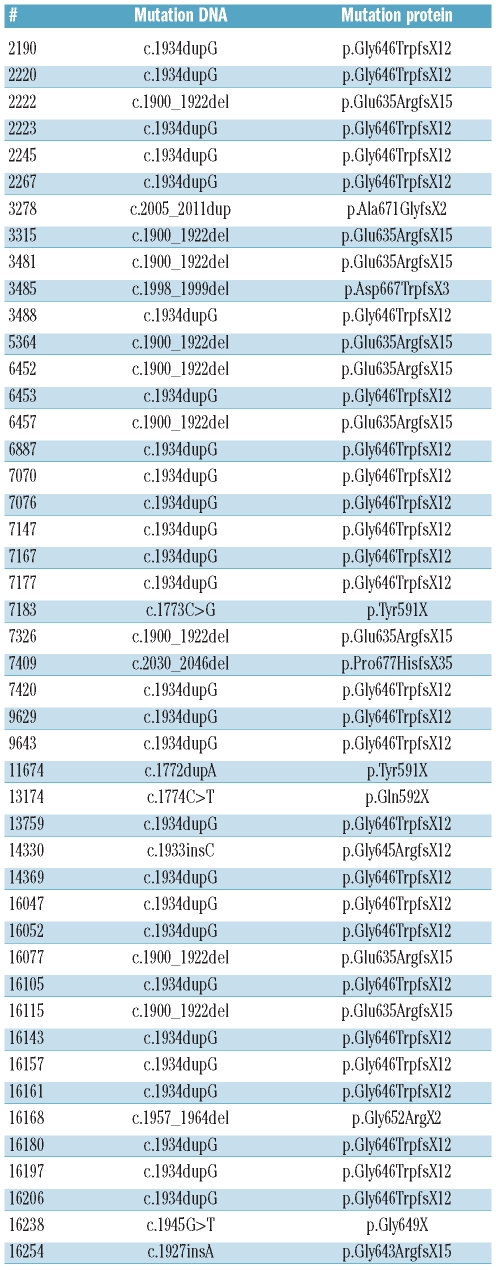
Information on the ASXL1 mutation status of all AML cases is available in Table 1 and of all AML cases for whom gene expression profiling has been performed (www.ncbi.nlm.nih.gov/geo, accession number GSE6891).
Table 1.
Types of ASXL1 mutations in 46 cases of acute myeloid leukemia.
The relation between ASXL1 mutations and various patients’ characteristics were determined by Student’s t-test, equal variances not assumed (continuous variables), Wilcoxon’s rank order test (continuous variables not normally distributed) and Fisher’s exact test (categorical variables).
We distinguished the following cytogenetic risk categories: (I) favorable, t(8;21), inv(16) or t(15;17); (II) unfavorable, inv(3)/t(3;3), t(6;9), 11q23 abnormalities other than t(9;11), -5, 5q-, -7, 7q-t(9;22) or monosomal karyotypes (MK); (III) intermediate risk, cytogenetically normal (CN) and the remaining AML cases (CA rest).
Overall survival (OS) end points were death (failure) and alive at last follow up (censored), as measured from entry onto trial. Relapse-free survival (RFS) end points were relapse, or death from any cause, measured from the date of achievement of CR. Distribution estimations and survival distributions of OS and RFS were calculated by the Kaplan-Meier method and the log rank test.
Results and Discussion
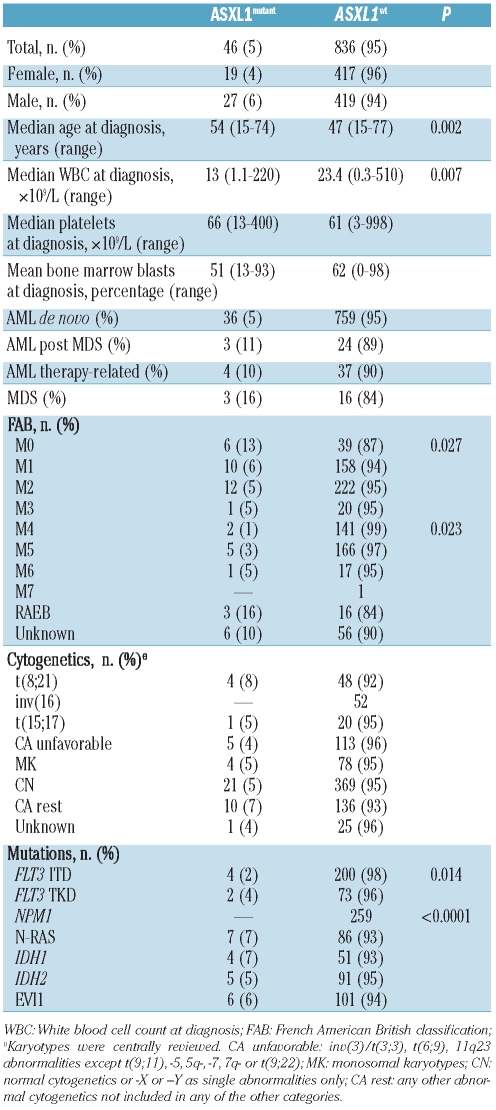
ASXL1 mutations were determined in 882 cDNA samples of AMLs by RT-PCR/dHPLC followed by direct sequencing (Table 1). ASXL1mut were identified in 46 AML cases (5.3%): in 35 out of 775 de novo AML cases (4.5%), in 3 out of 24 post-MDS AML cases (11%), and in 4 out of 37 therapy-related AML cases (10%) (Table 2).
Table 2.
Distribution of ASXL1 mutations in 882 cases of acute myeloid leukemia..
All the mutations found were frame shift mutations that caused a disruption of the PDH domain, and the most prevalent was p.Gly646TrpfsX12 (25 of 46, 54%). Recently, the ASXL1 p.Gly646TrpfsX12 mutation had been suspected to represent a PCR artifact rather than a somatic mutation.18 In the latter report, buccal samples from AML patients with this mutation all showed the ASXL1 mutation p.Gly646TrpfsX12. Furthermore, this particular mutation has also been reported in approximately 25% of healthy individual samples.18 For this reason, we set out to confirm the ASXL1 p.Gly646TrpfsX12 mutation in all our 25 samples, i.e. in a second independent sample of the same patient. Furthermore, in 2 available remission samples of these AML patients, the p.Gly646TrpfsX12 mutation in ASXL1 was absent. We also found the mutation in the Kasumi-1 cell line with the translocation t(8;21). In addition, we screened 91 samples from healthy individuals, and none of them revealed a mutation in exon 12 of ASXL1. Based on these results, we regard the p.Gly646TrpfsX12 mutation as a real somatic mutation.
ASXL1mut AML were more frequent at older age (P=0.004) and present with lower WBC count than AML with ASXL1 wild type (ASXL1wt) (P=0.014) (Table 2). ASXL1mut AML was associated with FAB M0 subtype (P=0.027), but inversely related to FAB M4 subtype (P=0.023), NPM1 mutations (P<0.0001; NPM1mut) and FLT3ITD (P=0.014) (Table 2).
To investigate the prognostic value of ASXL1mut, 807 patients with non-APL AML and those under 65 years of age were considered for survival analysis. The median follow up of these patients was 46.0 months (range 1.9–224 months). In this cohort, univariate survival analyses with several known recurrent aberrations in AML demonstrated that FLT3ITD (P<0.0001, unfavorable), NPM1mut (P=0.025, favorable), IDH2mut (P=0.02, favorable) and EVI1 overexpression (P<0.001, unfavorable) were significantly associated with OS, whereas FLT3TKD (P=0.214), NRASmut (P=0.53), and IDH1mut (P=0.81) were not.
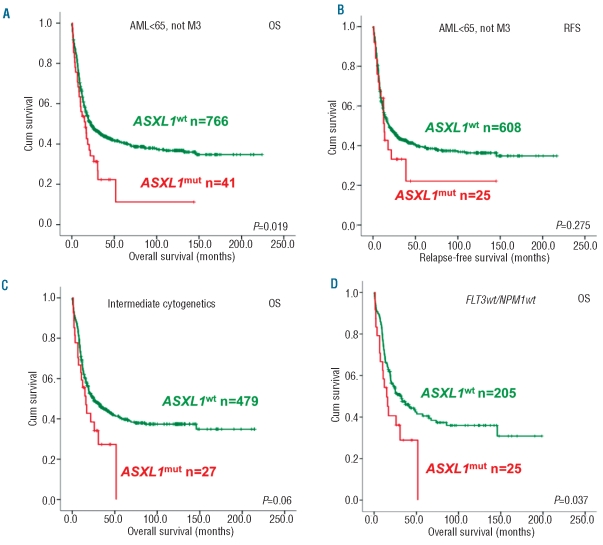
The complete response (CR) rate was significantly reduced in patients with ASXL1mut (61% vs. 79.6%; P=0.004). The median OS of patients with AML with or without ASXL1mut was 15.9 months vs. 22.3 months (P=0.019; Figure 1A). The relapse free survival was similar between the two groups once they achieved a CR (P=0.27; Figure 1B).
Figure 1.
Kaplan-Meier survival analyses of patients with AML with or without ASXL1 mutations. (A) OS for all AML patients (<65 years). (B) RFS for all AML patients (<65 years). (C) OS for patients with intermediate-risk AML. (D) OS for patients with intermediate-risk and FLT3wt and NPM1wt. Survival curves in red refer to cases with ASXL1mut; those in green to ASXL1wt. The log rank P value is indicated per Kaplan-Meier analysis.
In multivariate analyses, trial-stratified and considering other prognostic variables that were significantly associated with OS in univariate analysis, such as age, WBC, cyto-genetics, NPM1mut, FLT3ITD, IDH2mut and EVI1 overexpression, the presence of ASXL1mut was an independent prognostic factor for poor OS (HR 1.64, 95% CI= 1.12 – 2.4, P=0.010). Recently, Chou et al.7 were unable to demonstrate that ASXL1 mutations were an independent factor for prognosis in AML. Apart from population differences, this is likely due to the lower number of patient samples, both ASXL1 wild-type and mutant, in the survival analyses and the inclusion of other covariables in the multivariate analyses, such as RUNX1 mutations, which in fact appeared to be associated with ASXL1 mutations.7 When comparing both studies, it should be noted that the hazard ratios are similar, but that the confidence interval in the study by Chou et al.7 is greater as a result of decreased power. It is worthy of note that Chou and co-workers indicate that although ASXL1 mutations in their analysis did not represent a significant independent factor for prognosis, it is highly associated with a worse survival and lower CR rate.
The subgroup of AML patients with intermediate-risk cytogenetics showed a trend for worse OS for those with ASXL1mut AML (median OS: 15.9±7.4 vs. 24.7±7.9 months; P=0.06; Figure 1C), whereas there is no association between OS and the ASXL1mut genotype in normal karyotype AML (P=0.36). The trend for inferior OS in intermediate-risk cytogenetics, is significantly worse when AML patients with FLT3 wild-type (FLT3wt) and NPM1 wild-type (NPM1wt) genotypes are considered (median OS: 14.9±7.2 vs. 32.3±17.7 months; P=0.037; Figure 1D). In the other FLT3/NPM1 composite genotypes, the frequencies of ASXL1mut AML are too low to allow a meaningful analysis of the prognostic value of ASXL1mut.
The current study demonstrates that ASXL1 gene mutations are common in AML. These mutations are inversely associated with FLT3ITD, and mutually exclusive with NPM1mut. The presence of ASXL1mut in AML is an independent poor risk factor for OS mostly due to a higher initial resistance to chemotherapy. The adverse prognostic impact of ASXL1mut was confirmed in the subset of patients harboring intermediate-risk cytogenetics and lacking NPM1 and FLT3 mutations, and might contribute to refine prognosis in this subgroup of patients with uncertain outcome.
Acknowledgments
This study was performed within the framework of CTMM, the Center for Translational Molecular Medicine.
Footnotes
Funding: Leukemia BioCHIP project (grant 03O-102) and supported by the Dutch Cancer Society (Koningin Wilhelmina Fonds) and a grant (CM08/00027) from Instituto de Salud Carlos III (ISCIII).
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Fisher CL, Randazzo F, Humphries RK, Brock HW. Characterization of Asxl1, a murine homolog of Additional sex combs, and analysis of the Asx-like gene family. Gene. 2006;369:109–18. doi: 10.1016/j.gene.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 2.Fisher CL, Pineault N, Brookes C, Helgason CD, Ohta H, Bodner C, et al. Loss-of-function Additional sex combs like 1 mutations disrupt hematopoiesis but do not cause severe myelodysplasia or leukemia. Blood. 2010;115(1):38–46. doi: 10.1182/blood-2009-07-230698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher CL, Berger J, Randazzo F, Brock HW. A human homolog of Additional sex combs, ADDITIONAL SEX COMBS-LIKE 1, maps to chromosome 20q11. Gene. 2003;306:115–26. doi: 10.1016/s0378-1119(03)00430-x. [DOI] [PubMed] [Google Scholar]
- 4.Gelsi-Boyer V, Trouplin V, Adelaide J, Bonansea J, Cervera N, Carbuccia N, et al. Mutations of polycomb-associated gene ASXL1 in myelodysplastic syndromes and chronic myelomonocytic leukaemia. Br J Haematol. 2009;145(6):788–800. doi: 10.1111/j.1365-2141.2009.07697.x. [DOI] [PubMed] [Google Scholar]
- 5.Carbuccia N, Murati A, Trouplin V, Brecqueville M, Adelaide J, Rey J, et al. Mutations of ASXL1 gene in myeloproliferative neoplasms. Leukemia. 2009;23(11):2183–6. doi: 10.1038/leu.2009.141. [DOI] [PubMed] [Google Scholar]
- 6.Gelsi-Boyer V, Trouplin V, Roquain J, Adelaide J, Carbuccia N, Esterni B, et al. ASXL1 mutation is associated with poor prognosis and acute transformation in chronic myelomonocytic leukaemia. Br J Haematol. 2010;151(4):365–75. doi: 10.1111/j.1365-2141.2010.08381.x. [DOI] [PubMed] [Google Scholar]
- 7.Chou WC, Huang HH, Hou HA, Chen CY, Tang JL, Yao M, et al. Distinct clinical and biological features of de novo acute myeloid leukemia with additional sex comb-like 1 (ASXL1) mutations. Blood. 2010;116(20):4086–94. doi: 10.1182/blood-2010-05-283291. [DOI] [PubMed] [Google Scholar]
- 8.Boultwood J, Perry J, Pellagatti A, Fernandez-Mercado M, Fernandez-Santamaria C, Calasanz MJ, et al. Frequent mutation of the polycomb-associated gene ASXL1 in the myelodysplastic syndromes and in acute myeloid leukemia. Leukemia. 2010;24(5):1062–5. doi: 10.1038/leu.2010.20. [DOI] [PubMed] [Google Scholar]
- 9.Lowenberg B, Boogaerts MA, Daenen SM, Verhoef GE, Hagenbeek A, Vellenga E, et al. Value of different modalities of granulocyte-macrophage colony-stimulating factor applied during or after induction therapy of acute myeloid leukemia. J Clin Oncol. 1997;15(12):3496–506. doi: 10.1200/JCO.1997.15.12.3496. [DOI] [PubMed] [Google Scholar]
- 10.Lowenberg B, van Putten W, Theobald M, Gmur J, Verdonck L, Sonneveld P, et al. Effect of priming with granulocyte colonystimulating factor on the outcome of chemotherapy for acute myeloid leukemia. N Engl J Med. 2003;349(8):743–52. doi: 10.1056/NEJMoa025406. [DOI] [PubMed] [Google Scholar]
- 11.Ossenkoppele GJ, Graveland WJ, Sonneveld P, Daenen SM, Biesma DH, Verdonck LF, et al. The value of fludarabine in addition to ARA-C and G-CSF in the treatment of patients with high-risk myelodysplastic syndromes and AML in elderly patients. Blood. 2004;103(8):2908–13. doi: 10.1182/blood-2003-07-2195. [DOI] [PubMed] [Google Scholar]
- 12.Lowenberg B, Pabst T, Vellenga E, van Putten W, Schouten HC, Graux C, et al. Cytarabine dose for acute myeloid leukemia. N Engl J Med. 2011;364(11):1027–36. doi: 10.1056/NEJMoa1010222. [DOI] [PubMed] [Google Scholar]
- 13.Abbas S, Lugthart S, Kavelaars FG, Schelen A, Koenders JE, Zeilemaker A, et al. Acquired mutations in the genes encoding IDH1 and IDH2 both are recurrent aberrations in acute myeloid leukemia: prevalence and prognostic value. Blood. 2010;116(12):2122–6. doi: 10.1182/blood-2009-11-250878. [DOI] [PubMed] [Google Scholar]
- 14.Care RS, Valk PJ, Goodeve AC, Abu-Duhier FM, Geertsma-Kleinekoort WM, Wilson GA, et al. Incidence and prognosis of c-KIT and FLT3 mutations in core binding factor (CBF) acute myeloid leukaemias. Br J Haematol. 2003;121(5):775–7. doi: 10.1046/j.1365-2141.2003.04362.x. [DOI] [PubMed] [Google Scholar]
- 15.Valk PJ, Bowen DT, Frew ME, Goodeve AC, Lowenberg B, Reilly JT. Second hit mutations in the RTK/RAS signaling pathway in acute myeloid leukemia with inv(16) Haematologica. 2004;89(1):106. [PubMed] [Google Scholar]
- 16.Verhaak RG, Goudswaard CS, van Putten W, Bijl MA, Sanders MA, Hugens W, et al. Mutations in nucleophosmin (NPM1) in acute myeloid leukemia (AML): association with other gene abnormalities and previously established gene expression signatures and their favorable prognostic significance. Blood. 2005;106(12):3747–54. doi: 10.1182/blood-2005-05-2168. [DOI] [PubMed] [Google Scholar]
- 17.Gröschel S, Lugthart S, Schlenk RF, Valk PJ, Eiwen K, Goudswaard C, et al. High EVI1 expression predicts outcome in younger adult patients with acute myeloid leukemia and is associated with distinct cytogenetic abnormalities. J Clin Oncol. 2010;28(12):2101–7. doi: 10.1200/JCO.2009.26.0646. [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Wahab O, Kilpivaara O, Patel J, Busque L, Levine RL. The most commonly reported variant in ASXL1 (c.1934dupG;p.Gly646TrpfsX12) is not a somatic alteration. Leukemia. 2010;24(9):1656–7. doi: 10.1038/leu.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]