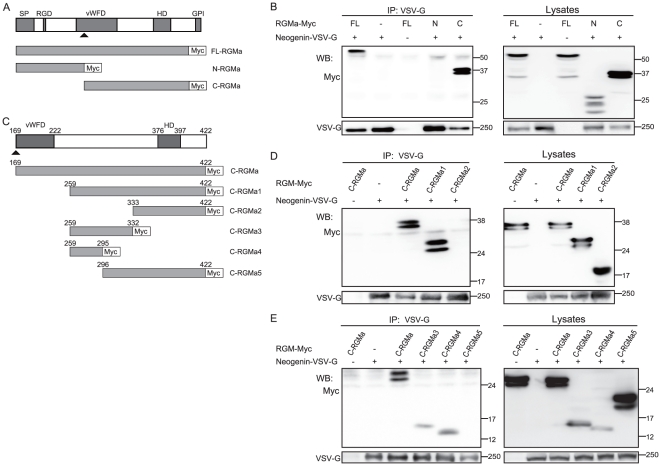
Figure 1. The RGMa domain required for binding to neogenin is within aa 259–295.
(A and C) Schematic representation of RGMa and its deletion mutants are shown with their domain structures. Arrowhead shows potential cleavage site. SP: signal peptide, vWD: von Willebrand factor type-D domain, HD: hydrophobic domain, GPI: GPI-anchor. (B, D, and E) Co-immunoprecipitation of full-length neogenin-VSV-G with the deletion mutants of RGMa-Myc. HEK293T cells were transiently transfected with the indicated plasmids. Cell lysates were immunoprecipitated with the anti-VSV-G antibody. The immunoprecipitates (IP) and cell lysates (Lysates) were analyzed by western blotting with anti-Myc and anti-VSV-G antibodies.