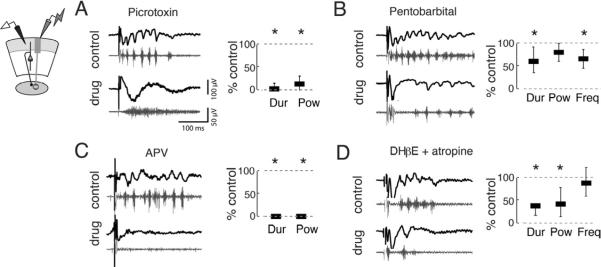
Figure 3. Multiple neurotransmitter systems contribute to gamma oscillations in the OT.
(Left panels) Top, black traces show representative traces bandpass filtered between 5–200 Hz, and the bottom, grey traces show the traces highpass filtered above 250 Hz.
(Right panels) Power, frequency, and duration were computed using LFPs filtered from 25–50 Hz. Bars represent medians and whiskers represent 25th and 75th thickness has no meaning. All drugs were applied to the bath. All p-values calculated with Friedman tests and are Bonferroni corrected for multiple comparisons.
A) (Far left) Schematic illustrating recording configuration in sOT. (Middle, Right) Picrotoxin (PTX, 10 μM) converted gamma oscillations to episodes of high frequency spiking. Duration (2.9% of control) in the gamma band and gamma power (13.7% of control) of oscillations were significantly reduced (p<0.001, n=6 slices).
B) GABAA–R enhancer pentobarbital (10 μM) significantly reduced the duration (60.2% of control) and frequency (69.8% of control) of oscillations (p<0.001, n=6), but did not alter gamma power (p=0.14).
C) NMDA-R antagonist APV (20 μM) abolished gamma oscillations. Both duration and power of gamma oscillations were eliminated (both are 0% of control, p<0.001, n=4).
D) ACh-R antagonists DHβE (40 μM) and atropine (5 μM) significantly reduced the duration (44.4% of control) and gamma power (61.2% of control) of oscillations (p<0.001, n=6). Frequency (87.9% of control) of gamma oscillations was unaffected (p>0.9).