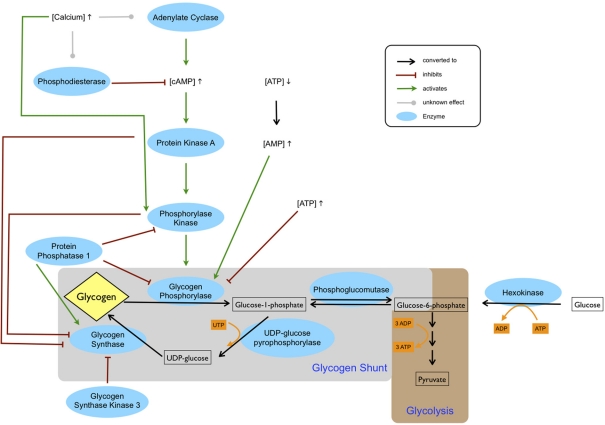
Figure 1.
Regulation of glycogen metabolism. The cAMP level is increased by adenylate cyclase and decreased by phosphodiesterase. Depending on the isoforms of these two enzymes, calcium can be either inhibiting or activating. An increased cAMP level leads to activation of protein kinase A, which in turn activates phosphorylase kinase. Phosphorylation by phosphorylase kinase, which requires calcium for activation, stimulates glycogen phosphorylase. Glycogen phosphorylase is also allosterically inhibited by ATP and activated by AMP, which accumulates during ATP depletion. Glycogen synthase is deactivated through phosphorylation by protein kinase A, phosphorylase kinase and glycogen synthase kinase 3. Glycogen synthase is activated by protein phosphatase 1, which inhibits phosphorylase kinase and glycogen phosphorylase. Glucose entering the cell is phosphorylated by hexokinase to glucose-6-phosphate and subsequently enters either glycolysis or the glycogen shunt, the latter constituting metabolism of glucose via glycogen. In glycogenesis one ATP equivalent in form of UTP is used by UDP-glucose pyrophosphorylase. Thus, glycogenolysis and subsequent glycolysis of glucose-6-phosphate entail a net gain of two ATP, whereas glycolysis of glucose-6-phosphate derived directly from glucose produces three ATP.