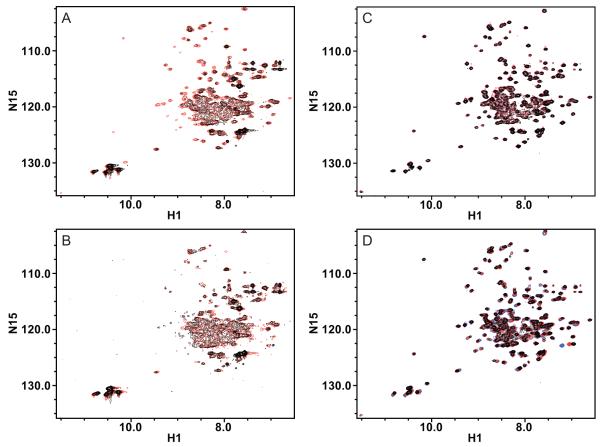
Figure 1.
Overlay of (1H, 15N)-TROSY HSQC spectra of TPP+-bound EmrE in isotropic bicelles reconstituted by different methods. A) 15N-EmrE in DMPC/DHPC bicelles is better resolved and has better signal/noise when reconstituted through liposomes (red) than when reconstituted through detergent exchange (black). B) The spectral quality of 15N-EmrE in DMPC/DHPC bicelles degrades with time for EmrE reconstituted via detergent. After nearly two weeks at 45°C, the spectrum (black) has less signal than the initial spectrum (red). C) The spectrum of 2H/15N/13C-EmrE reconstituted into DLPC/DHPC bicelles via liposomes (red) is virtually unchanged after more than six months (black). D) 2H/15N-EmrE can be stably reconstituted into isotropic bicelles made with different chain lengths (DLPC, blue; DMPC, red; DPPC, black), all capped by DHPC. The minor peak shifts indicate only small changes in EmrE structure with change in bicelle thickness. All spectra were collected at 45°C, in 20 mM KPi, 20 mM NaCl, 30 mM sodium cacodylate, pH 7.