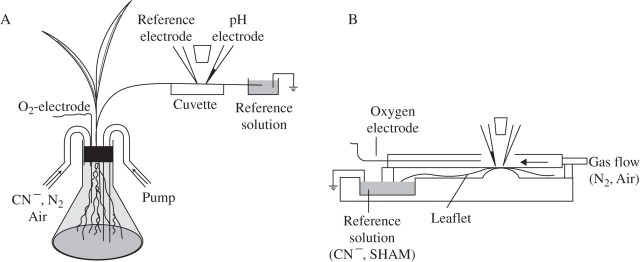
Fig. 1.
Principle of apoplastic pH measurements. (A) On a leaf of an intact plant. The roots were placed in an Erlenmeyer flask. The leaf was fixed with double-adhesive tape on a dry Plexiglass cuvette beneath the electrodes. The (cut) leaf tip rested in a chamber that held the exchangeable reference solution, but also harboured the earth electrode. Exchange of fluid (e.g. CN−) or gas flow (air, N2) was achieved through pipes while recording. An O2-minielectrode constantly controlled the O2. (B) On a detached leaf. The cuvette consists of two chambers: a wet (reference) chamber, which holds an exchangeable solution and the earth electrode. Added agents (e.g. FC or cyanide) were transported to the site of measurement through the transpiration stream. The leaf blade was fixed and the electrodes were placed in the dry chamber, as described in (A). An extra gas-flow cuvette with a small opening at the top to allow the approach of the electrodes could be placed on top of the leaflet. As in (A), an oxygen electrode constantly measured the O2.