FIG. 3.
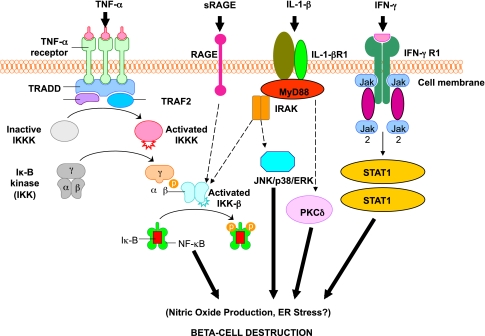
Activation of inflammatory mediators in pancreatic β-cells in type 1 diabetes. Tumor necrosis factor-α (TNF-α), IL-1β, and interferon-γ (IFN-γ) are the most likely cytokines acting in synergy during inflammation of pancreatic β-cells, leading to the activation of a final common pathway, such as nuclear factor-κB (NF-κB) and, ultimately, to β-cell destruction. NF-κB can be activated by a variety of stimuli, including TNF-α, IL-1, receptor for advanced glycation end products (RAGE), and Toll-like receptors (TLRs). IL-1β is an inflammatory cytokine that plays a major role in immune-mediated β-cell destruction. Interestingly, in patients with type 2 diabetes, the IL-1 pathway blockade with an IL-1 receptor antagonist (Anakinra) improved glycemic control and β-cell secretory function and resulted in a significant reduction marker of systemic inflammation, namely, C-reactive protein and IL-6 (56). A recent clinical study indicated that the blockade of the IL-1β pathway in type 1 diabetes resulted in the reduced ability of mononuclear cells to traffic to sites of inflammation (57). The latter observations provide evidence for a possible mechanistic link between type 1 and type 2 diabetes, and additional studies are necessary to unravel the common inflammatory pathways involved in the pathologic etiology of these two diseases. Compelling evidence indicates that cytokines influence the expression of inducible NO synthase (iNOS) leading to NO production. IL-1β and IFNγ, by NO synthesis, were reported to markedly decrease sarco(endo)plasmic reticulum Ca2+ ATPase 2b (SERCA2b) protein expression, deplete Ca2+ stores, and activate ER stress pathway, which is a potential contributing mechanism to β-cell death. Furthermore, cytokine-induced (IL-1β + IFN-γ) apoptosis of INS-1 cells appears to depend on NO production, as demonstrated by the use of the NO dioxygenase blocker NG-methyl-l-arginine. NO also contributes to cytokine-induced apoptosis through potentiation of Jun NH2-terminal kinase (JNK) activity and suppression of Akt/protein kinase B. Although whether oxidative stress plays a key role in the pathogenesis of type 1 diabetes is still being discussed, a reduced antioxidant capacity has been demonstrated in patients with type 1 diabetes compared with healthy control subjects. To summarize the cytokine signaling, TNF-α signals through trimerized p60 receptors that interact with the TNF receptor type 1–associated death domain protein (TRADD). Fas-associated protein with death domain (FADD) is then recruited by TRADD, thus allowing binding of receptor-interacting protein (RIP) and TNF receptor–associated factor 2 (TRAF2) to the receptor complex. TRAF2 activates NF-κB through NF-κB–inducing kinase (NIK)–inhibitor of κB kinase (IKK) and activates the JNK/p38 pathways. TNF-α is an inflammatory cytokine that appears to be associated with a number of autoimmune disorders, including type 1 diabetes. TNF-α may activate intraislet resident macrophages, resulting in the release of IL-1β, which generates iNOS expression and the overproduction of NO in β-cells. Alterations in the number and function of CD4+CD25+ T-cells may be an additional mechanism by which TNF-α may cause type 1 diabetes in NOD mice. The role of RAGE mediated by NF-κB has not been entirely elucidated, although RAGE may be an important intermediary in causing monocyte production of inflammatory mediators such as TNF-α. It is possible that increased expression of RAGE in response to hyperglycemia may lead to activation of innate and even adaptive immune responses and enhance β-cell destruction. After IL-1β binding to IL-1βR1, MyD88 is recruited to the receptor complex. MyD88 interacts with IL-1 receptor–associated kinase (IRAK), allowing the binding of TRAF6 to IRAK. TRAF6 causes activation of mitogen-activated protein kinase/stress-activated protein kinase and activation of the NF-κB pathway by transforming growth factor-β–activated kinase 1 (TAK1)–mediated activation of IKK. IL-1β also stimulates activation of protein kinase C-δ (PKC-δ), possibly through phospholipase C generation of diacylglycerol. ERK, extracellular signal–regulated kinase; Jak, Janus kinase; STAT1, signal transducer and activator of transcription-1.