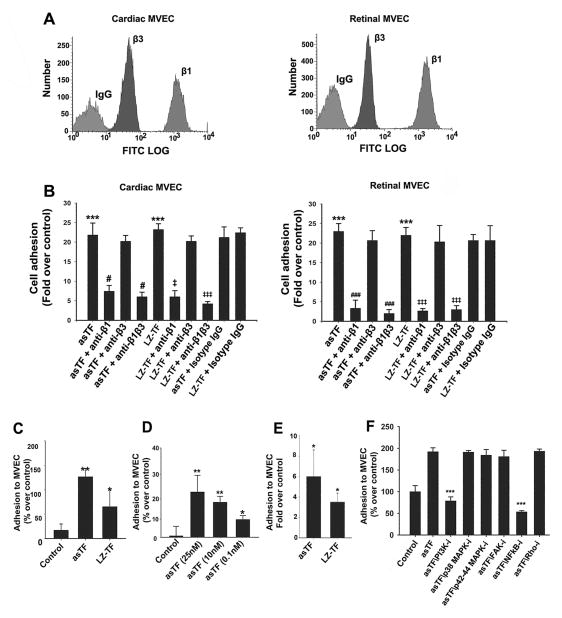
Fig 4.
LZ-TF and asTF ligate β1 integrins on human MVEC and promote MVEC-monocyte interactions. (A). Flow cytometry, relative levels of β1/β3 integrins on the surfaces of cardiac and retinal MVEC. (B) MVEC adhesion to LZ-TF/asTF (n≥3). Values are mean ± SD; *** p<0.001 compared to BSA; # and ### p<0.05 and p<0.001, respectively, compared to asTF; ‡ and ‡‡‡ p<0.05 and p<0.001, respectively, compared to LZ-TF. (C) LZ-TF and asTF promote MVEC-PBMC interactions in the orbital shear assay. (D) Dose-response experiments, asTF in the orbital shear assays employing PBMC. (E) LZ-TF and asTF promote MVEC-PBMC interactions in the laminar flow assay – (F) Effects of kinase inhibition on asTF-triggered MVEC-monocyte interactions under orbital shear; MVEC were pre-incubated with PI3 kinase inhibitor LY294002 (10 μM), p38 MAPK inhibitor SB203580 (10 μM), p42/p44 MAPK inhibitor PD98059 (20 μM), FAK II inhibitor (10 μM), NFkB inhibitor BMS-345541 (10 μM), and Rho kinase inhibitor (10 μM). *p<0.01, **p<0.001, ***p<0.0001. n≥3 for each assay, the values are mean ± SD. # p<0.05 compared to asTF. ‡ and ‡‡ p<0.05 and p<0.01, respectively, compared to LZ-TF.