Figure 4.
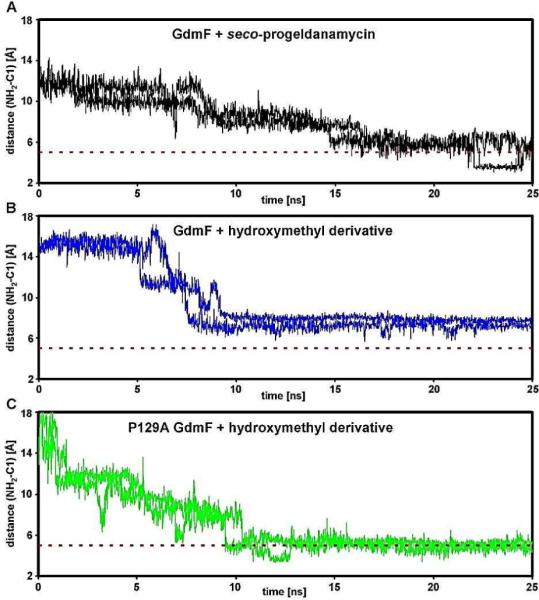
Restrained MD simulations of the aromatic ring whilst penetrating the catalytic site of GdmF (and its variant P129A) using a distance restraint (5 Å) with linearly increasing energy penalty between C-1 and C-19, the latter being located halfway between the nucleophilic groups (–NH2, –OH or –CH2OH) decorating the aromatic ring (Figure 3). Using this approach we were able to qualitatively probe the conformational space available to the aromatic ring and thus the distance of the nucleophilic groups to C-1. The dashed red line indicates close proximity of the NH2 nucleophile to C-1 after penetration as observed for the natural ligand seco-progeldanmycin (in black). In contrast, the approach of the NH2 nucleophile of the hydroxymethyl derivative of seco-geldanamycin towards C-1 appears hindered (in blue) which might explain observed slower lactam cyclization rates for this non-natural substrate. In fact, detailed analyses of the dynamic trajectories imply that the naturally occurring compound is still mobile within the catalytic pocket of GdmF, while the hydroxymethyl derivative shows static properties (compare Figure 4A and 4B and data not shown). The static ligand properties can be overcome once proline 129 is mutated to an alanine (P129A, in green) rationalizing the observed turnover specificity for Asm9 (compare Figure 4B and 4C). Note that P129A mimics the catalytic pocket of Asm9 (see Figures 2,3)).
