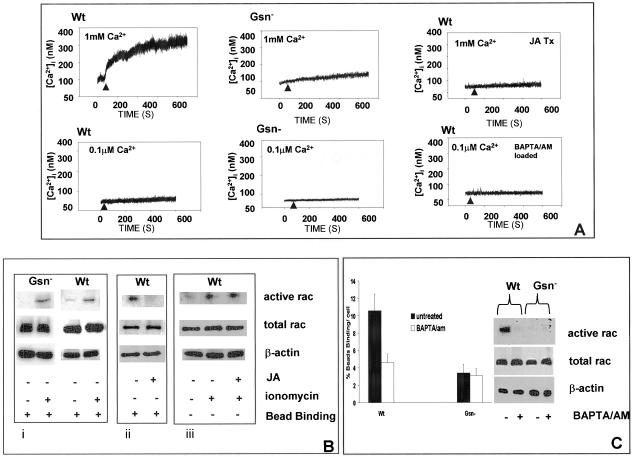
Figure 6.
Role of calcium in rac activation. (A) Intracellular calcium transients were measured in single, fura 2/AM (3 μM)-loaded cells, detected by ratio fluorimetry at 346 nm/380 nm (excitation) and 510 nm (emission) in the presence (1 mM) or absence (0.1 μM) of CaCl2. Loaded cells were plated on collagen-coated beads and measurements were done after initial cell-bead binding and only in cells with attached beads. WT cells show robust calcium transients compared with Gsn- cells. To determine the importance of subcortical actin filaments on collagen-induced calcium increases, cells were incubated with JA (1 μM for 15 min) and during attachment to collagen beads, [Ca2+]i was measured by ratio fluorimetry. WT cells were also loaded with BAPTA/AM (3 μM in buffer containing 0.1 μM CaCl2) and measured by ratio fluorimetry. (B) Role of calcium in regulation of rac. i) Gsn- and WT cells were treated with ionomycin (2 μM; indicated by +) for 20 min, and rac activation was measured with PAK binding assay in the presence of collagen beads as described in Figure 4. ii) Effect of actin polymerization on activation of rac measured after bead binding in the JA- and vehicle-treated WT cells. iii) JA-treated WT cells were further treated with or without ionomycin showing effect on rac activation. (C) Collagen bead binding was measured in WT and Gsn- cells in low calcium buffer (0.1 μM CaCl2) with and without preincubation with BAPTA/AM as described above. Rac activation assays were performed as in B with or without BAPTA/AM preincubation.