Figure 2.
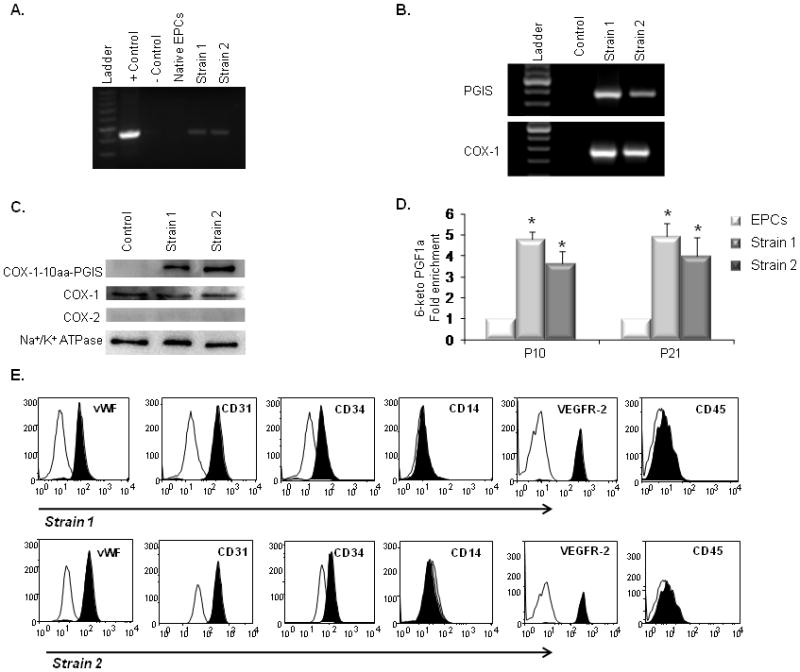
Characterization of PGI2-EPC strains that overexpress active triple-catalytic enzyme. (A) PCR showed the integration of the COX-1-10aa-PGIS transgene into genomic DNA in strain 1 and 2 EPCs. The plasmid encoding COX-1-10aa-PGIS was used as the positive control; an empty vector served as the negative control. (B) RT-PCR detected human COX-1 and PGIS transcripts in rat EPC strains 1 and 2. (C) Western blots showed the overexpression of COX-1-10aa-PGIS fusion protein (130 kD) in strain 1 and 2 EPCs. PGI2-EPC strains expressed endogenous COX-1 at a similar level as native EPCs (control). No endogenous COX-2 expression was detected by Western blot. Na+/K+ ATPase protein served as a loading control. (D) PGI2-EPC strains consistently produced significantly higher levels of PGI2 than native EPCs. To assess prostacyclin production, the metabolite 6-keto PGF1α was measured by enzyme immunoassay in the supernatants after cells were grown under endothelial conditions for 24 hours (n=3). Native EPCs served as a control in B–D. *P<0.05 compared to control. (E) Representative flow cytometry analysis showed that PGI2-EPC strains 1 and 2 retained similar expression levels of CD31, CD34, vWF, VEGFR-2, CD14, and CD45 as compared to those of native EPCs (displayed in Fig.1D), n=3.
