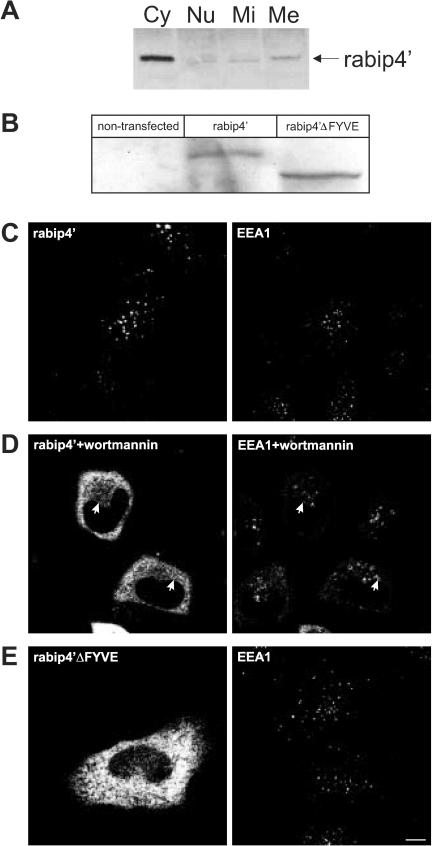
Figure 2.
(A) The FYVE domain is required for membrane binding of rabip4′. HEp-2 cell lysates were fractionated as described in MATERIALS AND METHODS. Cytosolic (Cy), nuclear (Nu), mitochondrial (Mi), and microsomal (Me) fractions were analyzed by Western blotting by using a rabbit antibody against rabip4′. HeLa cells were transfected with VSV-G-tagged rabip4′ (B-D) or N-RUN-CC13 mutant (rabip4′ΔFYVE) lacking the FYVE domain (B and E). Cell lysates were resolved by SDS-PAGE and analyzed by Western blotting with an antibody against the epitope tag. Both constructs are expressed at the same level (B). HeLa cells expressing VSVG-tagged rabip4′ (C) were incubated for 15 min at 37°C with 50 nM wortmannin (D). Cells were fixed 18-24 h after transfection, doubly labeled with mouse anti VSV-G and rabbit anti-EEA1, stained with Alexa488-labeled rabbit anti-mouse and Cy3-labeled goat anti-rabbit, and examined by fluorescence microscopy. Note extensive colocalization between rabip4′ and EEA1 in C. In wortmannin-treated cells, rabip4′ redistributed partially in the cytoplasm but also colocalized with EEA1 on enlarged endosomes (arrows in D). Double-label immunofluorescence of cells transfected with VSV-G-tagged N-RUN-CC13 and endogenous EEA1 shows that the construct lacking the FYVE domain is localized in the cytoplasm (E). Bar, 10 μm.