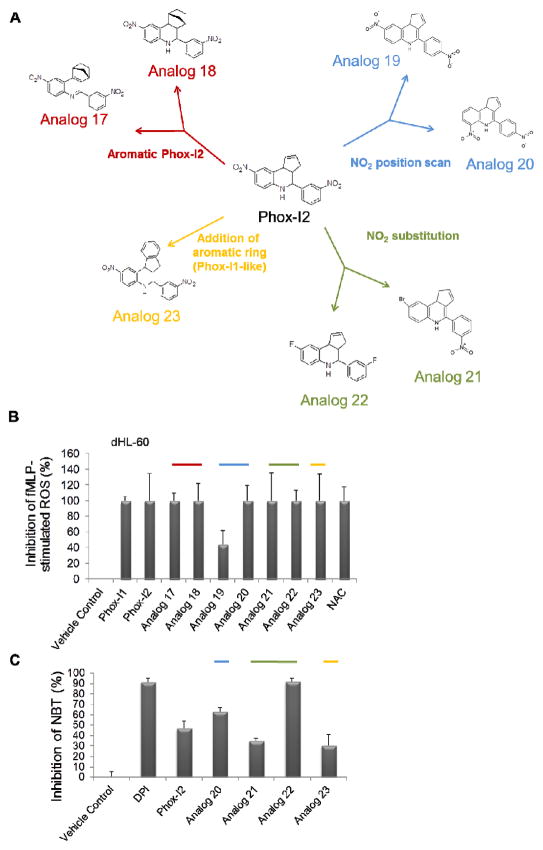
Figure 6. Medicinal chemistry optimization of Phox-I2 allows for the replacement of potentially toxic nitro groups.
(A) Compounds with similar structures to Phox-I2 were synthesized and broken down into 4 different categories; 1. NO2 position scan, 2. NO2 substitution, 3. Addition of an aromatic ring (rendering it similar to Phox1), 4. Aromatic Phox2. (B) DCFDA FACS analysis was performed using differentiated HL-60 cells treated for 2 hours with compounds from A prior to stimulation with fMLP. (C) Freshly isolated primary murine neutrophils were stimulated with fMLP to initiate ROS production, cells were then treated with DMSO control, DPI, Phox-I2, Analogs 20, 21, 22, 23. A Nitroblue tetrazolium assay was performed and imaged in order to quantitate superoxide inhibition. Non-fMLP treated ROS levels were subtracted prior to normalization to vehicle control treated sample.