Scheme 8.
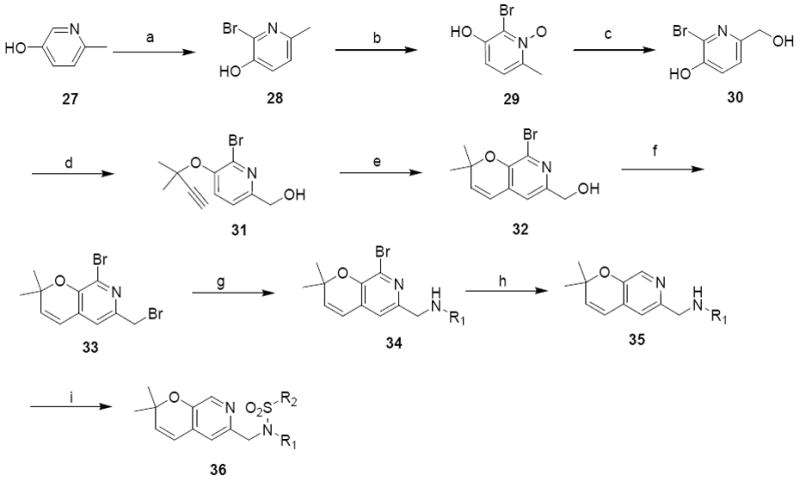
Synthesis of pyrano(2,3c) pyridine analoguesa
R1 = phenyl and R2 = 4-methoxyphenyl (36a), 4-nitrophenyl (36b)
R1 = cyclohexyl and R2 =4-isopropylphenyl (36c), 3,4-dimethoxyphenyl (36d)
a Reagents and Conditions: (a) Br2, pyridine, 0°C, 74%; (b) m-CPBA, THF, 70%; (c) 1. TFAA, 2. MeOH, 30%; (d) 3-chloro-3-methyl -1-butene, K2CO3, KI, CuCl2, acetone, 57%; (e) CuCl, toluene, microwave heating (200 W, 120 °C, 1 hour), 70%; (f) CBr4, PPh3, DCM, 40%; (g) DIEA, DMF, 60 - 78%; (h) BuLi, THF, -78°C, 50 – 70% (i) R2SO2Cl, pyridine, r.t., 70 – 89%.
