Table 1.
Development of the Tandem Dehydrogenation/Diels-Alder
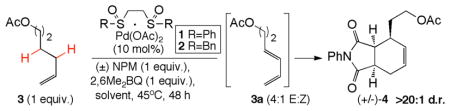 | ||||||
|---|---|---|---|---|---|---|
| entry | catalysta | solvent | additivef | dienophilee | yield dieneb | yield cycloadductc |
| 1 | Pd(OAc)2 | dioxane | ----- | ----- | <1d | ----- |
| 2 | 1 | dioxane | ----- | ----- | 6 | ----- |
| 3 | 2 | dioxane | ----- | ----- | 28 | ----- |
| 4 | 2 | dioxane | ----- | NPM | <1d | 33 |
| 5 | 2 | DCE | ----- | NPM | <1d | 52 |
| 6 | 2 | DCE | p-NO2BzOH | NPM | <1d | 74 |
| 7 | 2 | DCE | p-NO2BzOH | ----- | 35 | ----- |
10 mol% catalyst
Isolated after 24 hr as a 4:1 mixture of E/Z isomers along with rSM
isolated yield
Determined by GC analysis
NPM = N-phenylmaleimide (1.0 equiv.)
10 mol%
