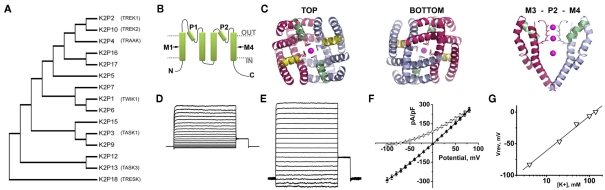
Figure 2.
The structure and function of K2P channels. Fifteen K2P channel subunits have been identified in humans. Two subunits come together to form a K+ selective permeation pathway that opens and closes with little or no voltage or time-dependence. (A) A phylogenetic tree calculated from the alignment of the 15 K2P proteins expressed in humans shows the relatedness of subunits. Functional expression has not been observed for K2P7, 12, and 15. The descriptive names of the K2P channels discussed in this review are also given. (B) A cartoon showing that K2P subunits are integral membrane proteins with internal amino (N) and carboxy (C) termini, four transmembrane domains, M1–M4 and two re-entrant pore forming loops, P1 and P2. (C) A structural model of the Drosophila K2PØ channel based on experimental constrains and homology to the crystal structure of the voltage-dependent channel, Kv1.2. The top and bottom of the model are shown, as well as a side view showing the arrangement of transmembrane M3, P2, and M4. Occupation of the pore by K+ is denoted in each case. From above or below, the model shows twofold symmetry, with conservation of the fourfold symmetry required to form a K+ selective pore. Adapted from Kollewe et al. (2009). (D) A K+ leak current recorded from a Chinese Hamster Ovary cell transfected to express active human K2P1 channels. The inside of the cell is perfused with 140 mM K+ and the outside of the cell is perfused with 4 mM K+. (E) The same cell recorded in (D) with 140 mM K+ on the inside and the outside of the cell. (F) The current–voltage relationships for the cell recorded in (D) ○, and (E) (▲). (G) The same cell recorded in (D) studied in various concentrations of external K+. The voltage where zero-current was passed for each condition is plotted against the log10 of the external K+ concentration. The data are fit to a linear regression and show a shift of ∼54 mV per 10-fold change in K+. This relationship is predicted by the Nernst equation and confirms the K+ selective nature of the channel (see also Figure 1A). Elements D to F are adapted from Plant et al. (2010).