Figure 3.
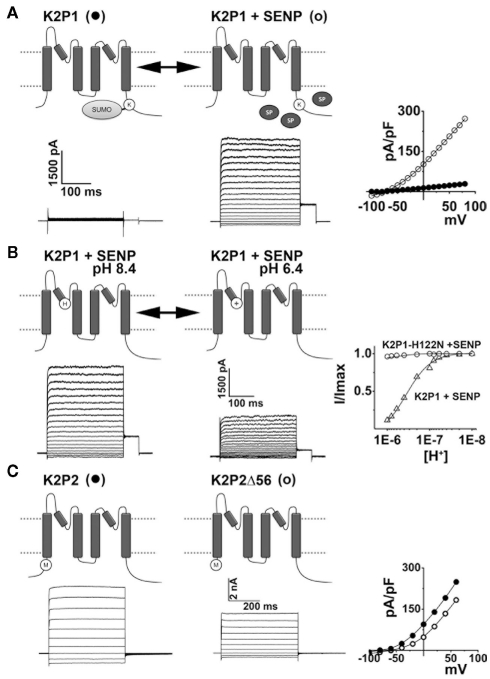
K2P channels are subject to regulation by diverse mechanisms. The operation of K2P channels is tightly controlled, both acutely and in the long-term, by a plethora of stimuli and regulatory pathways. (A) K2P1 channels are constitutively covalently modified at lysine residue 274 (K) by the small ubiquitin-like modifier protein, SUMO. Sumoylation silences K2P1 but is reversed by the action of SUMO-specific proteases (SENP, SP) to reveal active channels that mediate K+ selective leak currents in excitable cells such as neurons and cardiac myocytes. Example current-families and current–voltage relationships for K2P1 alone (●) and K2P1 in the presence of SENP (○) are shown; adapted from Plant et al. (2010). (B) Acid-sensitive K2P (TASK) channels have a histidine (H) adjacent to the K+ selectivity filter in the first P-loop. Thus, K2P3, K2P9, and active K2P1 channels pass currents that are reversibly blocked by protonation of this residue during acidification. Example currents-families for active K2P1 channels (in the presence of SENP) are shown at external pH 8.4 and 6.4. The proton-dependent block of active K2P1 channels is plotted (Δ) and shows that half-block occurs at pH 6.7. Active K2P1 channels in which the protonatable histidine is substituted for an asparagine residue are not sensitive to external acidification (○); adapted from Plant et al. (2010). K2P18 channels from rodents and other mammals are also acid-sensitive however, primate clones have a tyrosine rather than a histidine in the first P-loop and thus, human K2P18 channels are insensitive to acidification. (C) Full-length K2P2 channels mediate K+ selective leak currents. Alternative translation initiation of KCNK2 mRNA transcripts results in K2P2Δ, a subunits that lack the first 56 residues of the intracellular N-terminus. K2P2Δ is expressed throughout the brain and spinal cord at levels that change throughout development and pass smaller currents than full-length channels because of an increased permeability to Na+under physiological conditions. Example current-families and current–voltage relationships for full-length (●) and Δ56 variants of K2P2 (○) are shown; adapted from Thomas et al. (2008).