Summary
Aim. The aim of this work is to determine the incidence of ventilator-associated tracheobronchitis (VAT) and ventilator-associated pneumonia (VAP) and to define the define the most important respiratory pathogens in patients with inhalation injury. Introduction. Infectious complications in severely burned patients present serious problems. Patients with inhalation injuries are exposed to greater risk owing to the possible development of infectious complications in the lower respiratory tract. VAP is the predominant cause of death in these patients. This is due to the increasing resistance of strains of Gram-negative bacteria such as Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae. Design. Retrospective, monocentric. Setting. A five-bed burn intensive care unit. Material and methods. Between 2004 and 2009, 348 adult patients were hospitalized in the intensive care unit of the Department of Burns and Reconstructive Surgery, Brno University Hospital, Czech Republic. Of these, 127 (36.49%) were diagnosed by bronchoscopy as having inhalation injury. The prerequisite for inclusion in the cohort was an inhalation injury requiring artificial ventilation for at least 48 h. The lower airway microbiological condition was monitored regularly by sampling biological material for cultures (sputum, tracheobronchial aspirates, etc.). For the diagnosis of VAP and VAT we used the Centers for Disease Control and Prevention criteria and the Clinical Pulmonary Infection Score. Results. The average age of the 127 patients (31 women/96 men) included in the study was 38.4 yr (range, 21-69 yr) and the average total body surface area (TBSA) burned was 29.3% (range, 2-75%). The average length of hospital stay was 49.4 days (range, 4-150 days) and the duration of mechanical ventilation 8.7 days; 18 patients (14.2%) died. In patients with inhalation injury, 309 strains of bacteria were cultivated from the lower respiratory tract, of which 234 were Gram-negative. All of these bacterial strains were isolated in significant quantities for lower respiratory tract infection. The most common bacteria isolated from the lower respiratory tract was Klebsiella pneumoniae (78 times), followed by Pseudomonas aeruginosa (49x), and Acinetobacter baumannii (28x). VAT was diagnosed in 109 patients (85.8%) in the cohort. The incidence of VAT was calculated to be 98.8 per 1000 days of mechanical ventilation. VAP was diagnosed in 34 patients in the cohort (26.8%). The incidence of VAP was calculated as being 30.8 cases per 1,000 days of mechanical ventilation. In eight patients (23.5%), VAP was diagnosed within 5 days of initiation of mechanical ventilation (early onset) and in 26 patients (76.5%) after a longer period (late onset). The most common aetiological agent of VAT and VAP was Klebsiella pneumoniae (respectively 41.3% and 35.3%). Conclusion. In this study we were able to determine the incidence of VAP and VAT in patients with inhalation injury. In spite of the advances in diagnostics and therapy, inhalation injury is still burdened with disappointingly high morbidity and mortality rates. For this reason, the treatment of VAP remains a major challenge for all physicians caring for patients with inhalation injury.
Keywords: inhalation injury, ventilator-associated pneumonia, ventilator-associated tracheobronchitis, Gram-negative bacterial strains, resistance
Abstract
But. Les Auteurs de cette recherche se sont proposé de déterminer la fréquence de la trachéobronchite et de la pneumonie associées à la ventilation assistée et de définir les pathogènes respiratoires les plus importants chez les patients atteints de lésions dues à l'inhalation. Introduction. Chez les patients gravement brûlés les complications des infections présentent de graves problèmes. Les patients atteints de lésions dues à l'inhalation sont plus exposés à cause du risque majeur de l'évolution possible de complications infectieuses aux voies respiratoires inférieures. La pneumonie associée à la ventilation assistée est la principale cause de décès chez ces patients, à cause de la résistance toujours croissante des souches de bactéries à Gram négatif telles que Pseudomonas aeruginosa, Acinetobacter baumannii et Klebsiella pneumoniae. Typologie de la recherche: Rétrospectif, monocentrique. Cadre. Unité de soins intensifs à cinq lits pour les patients brulés. Matériel et méthode. Entre 2004 et 2009, 348 patients adultes ont été hospitalisés dans l'unité de soins intensifs du département de brûlures et chirurgie reconstructrice de l'hôpital universitaire de Brno. Chez 127 de ces patients (36,49%) la bronchoscopie a révélé des lésions par inhalation. La condition essentielle pour l'inclusion dans la cohorte prise en considération était la présence de lésions par inhalation qui nécessitait la ventilation artificielle pour au moins 48 h. L'état microbiologique des voies respiratoires inférieures a été monitoré par prélèvement de matériel biologique pour les cultures (crachats, ponctions trachéobronchique, etc.). Pour le diagnostic de la pneumonie et de la trachéobronchite associées à la ventilation assistée, les critères des Centres pour le Contrôle et la Prévention et le Score de l'Infection Pulmonaire Clinique ont été utilises. Résultats. L'âge moyen des 127 patients (hommes, 31; femmes, 96) pris en considération était de 38,4 ans (oscillation, 21-69 ans) et la surface corporelle moyenne brulée 29,3%. La durée moyenne de l'hospitalisation était de 49,4 jours (oscillation, 4-150 jours) et la durée moyenne de la ventilation mécanique 8,7 jours. Dix-huit patients (14,2%) sont décédés. Chez les patients atteints de lésions par inhalation, 309 souches de bactéries ont été cultivées à partir des voies respiratoires inférieures, dont 234 étaient à Gram négatif. Toutes ces souches bactériennes ont été isolées dans des quantités significatives pour l'infection des voies respiratoires inférieures. Les bactéries les plus communes isolées dans les voies respiratoires inférieures était Klebsiella pneumoniae (78 fois), suivie par Pseudomonas aeruginosa (49), et Acinetobacter baumannii (28). La trachéobronchite associée à la ventilation assistée (TVA) a été diagnostiquée chez 109 patients (85,8%) dans la cohorte. La fréquence de la TVA a été calculée comme 98,8 pour 1000 jours de ventilation mécanique. La pneumonie associée à la ventilation assistée (PVA) a été diagnostiquée chez 34 patients de la cohorte (26,8%). La fréquence de la PVA a été calculée 30,8 cas pour 1000 jours de ventilation mécanique. Chez huit patients (23,5%), la PVA a été diagnostiquée dans les cinq premiers jours après l'initiation de la ventilation mécanique (apparition précoce) et chez 26 patients (76,5%) après une période plus longue (apparition tardive). L'agent étiologique le plus commun de la TVA et de la PVA était Klebsiella pneumoniae (respectivement 41,3% et 35,3%). Conclusion. Cette étude a déterminé la fréquence de la PVA et de la TVA chez les patients atteints de lésions par inhalation. Malgré les progrès en matière de diagnostic et de thérapie, les lésions par inhalation sont toujours associées à un taux élevé de morbidité et de mortalité. Pour cette raison, le traitement de la PVA reste un défi important majeur pour tous les médecins qui soignent les patients atteints de lésions par inhalation.
Introduction
Advances in burn treatment, especially in the last three decades, have led to reduced mortality rates and modified the causes of death.1 Burn shock, which typically develops in patients with burns covering over 20% total body sur-face area (TBSA), was the predominant cause of death, especially between 1930 and 1940. Sophisticated fluid resuscitation can help avert this complication and reduce mortality. Septic complications caused by infections of the burned area are now solved by prompt necrectomy and immediate closure of the epithelial defect.
Inhalation injury is currently the leading cause of mortality in severely burned patients.2 Inhalation injury is defined as acute respiratory difficulty caused by inhalation of smoke from combustible materials.3 It is a common complication in burn treatment. Isolated inhalation injury can also have fatal consequences for the patient. If it occurs in connection with burn trauma, inhalation injury will result in longer hospitalization and increased morbidity and mortality.4We must suspect inhalation injury if the burn is localized in the head or neck, if the presence of soot is noted in the mouth or nose, if soot is present in the sputum, or if the patient exhibits subjective symptoms such as impaired swallowing or hoarseness. Inhalation of smoke from combustible materials can damage the respiratory tract at different levels. We can therefore further distinguish the following categories: trauma of the upper and lower respiratory tract, impaired pulmonary parenchyma, and the inhalation of small particles, which can lead to systemic effects (inhalation of cyanide, CO).5 Generally, the larger and more hydrophilic an inhaled particle is, the more it deposits in the upper airways (nasal). Thus, the size of the particle is very important for prediction of the point of maximum affection. Inhaled particles larger than 10 microns are retained in the nasal cavity and nasopharynx, while particles sized 3-10 micrometres reach the tracheobronchial tree. Particles sized 1-2 micrometres can then penetrate to the alveoli.6 Even smaller particles entering the airways may be exhaled without affecting the respiratory tract or other organs. Gases less soluble in water penetrate easily via alveolocapillary membranes and their effect can then be generalized. The diagnosis of inhalation injury is usually not difficult. The limitation today is particularly the absence of clear standards for the verification of inhalation injury. The most widely used method for diagnosing inhalation injury is the application of a fibre optic bronchoscope.7 Patients with inhalation injury are especially threatened by swelling of the airways, which occurs 24-36 h after the insult. It is therefore necessary to intubate the patient and initiate artificial (mechanical) ventilation. As the oedema progresses, it can become very difficult or sometimes even impossible to intubate the patient and many physicians therefore prefer prophylactic intubation in every patient suspected of inhalation injury. In patients with inhalation injury, the airways defence mechanisms are affected, which can lead to the development of infection. The most serious infectious complication that affects this group is VAP, which usually manifests itself 72 h after the insult.8 VAP is defined as nosocomial pneumonia in patients requiring mechanical ventilation for at least 48 h. Many authors distinguish VAP as primary endogenous, secondary endogenous, and exogenous. Primary endogenous VAP is not nosocomial pneumonia in the true sense but is caused by potentially pathogenic micro-organisms carried by the patient at the time of admission to the intensive care unit (ICU). Secondary endogenous potential pathogenic micro-organisms are present in the gastrointestinal tract (gut). They can under certain conditions migrate and cause an infection in the lower airways.
In typical exogenous nosocomial infection, bacteria come from the surrounding patients, the ventilator, hands of personnel, etc.). Risk factors for VAP are shown in Table I.
Table I. Risk factors for the development of ventilator-associated pneumonia.
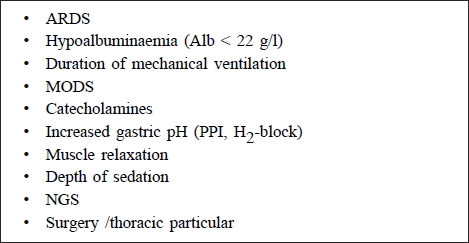
Risk factors for development of VAP9
The incidence of VAP varies between 5 and 50% of patients on mechanical ventilation, depending on the group. Patients with burns and inhalation injury have the highest incidence of VAP in all groups of critical patients. Generally, in patients on mechanical ventilation, the risk of developing VAP is 5% and increases by 1-2% with each successive day of mechanical ventilation.10 Optimal comparison of incidences of VAP bases the number on 1000 ventilation days. The National Nosocomial Infections Study rates of VAP varied from 5 cases per 1,000 days in paediatric patients to 35 cases per 1,000 days in patients with burns.11 More often, the number of VAP is about 15 cases per 1000 ventilation days. However, patients from a surgical ICU have a higher frequency than patients from a medical ICU.
In terms of prognosis and long-term survival, it is also important to know which pathogen is causing VAP. Compared with Gram-positive cocci, non-fermenting Gramnegative bacteria are related to a higher mortality rate.12 In contrast, the mortality rate is higher with non Candida albicans strains than with Candida albicans strains.13
Materials and methods
In the period 2004-2009, 348 adult patients were hospitalized in the ICU of the Department of Burns and Reconstructive Surgery, University Hospital Brno. In 127 of these patients (36.49%) inhalation injury was diagnosed by bronchoscopy. A prerequisite for inclusion in the cohort was the presence of an inhalation injury requiring artificial ventilation for at least 48 h. The microbiological situation of the lower airways was monitored regularly in all of the patients by sampling the biological material for culture (sputum, tracheobronchial aspirates, etc.). For the diagnosis of VAP and VAT, the Centers for Disease Control and Prevention criteria (Table II) and the Clinical Pulmonary Infection score were used (Table III).
Table II. Centers for disease control and prevention (CDC) criteria for VAP14.
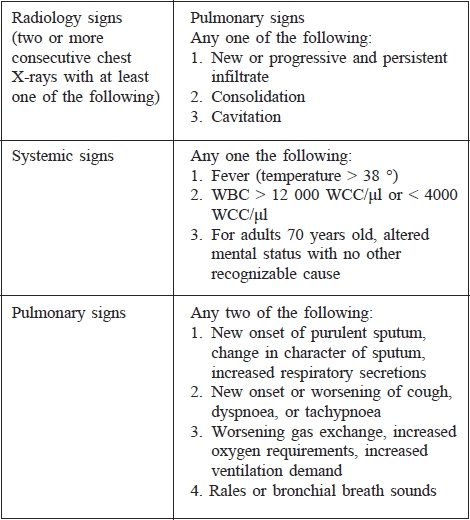
Table III. Calculation of Clinical Pulmonary Infection Score.
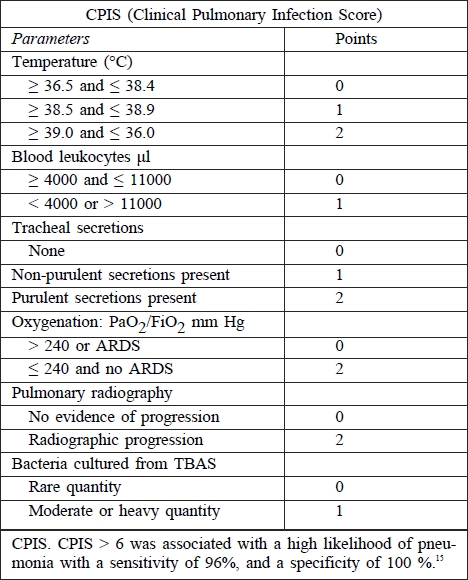
Diagnosis of pneumonia in patients requiring mechanical ventilation is often very difficult and is based on a combination of clinical and laboratory methods (microbiological, biochemical, haematological). Today there is still no clear standard for the diagnosis of VAP in patients with inhalation injury. Clinical diagnosis of VAP is usually based on the presence of fever (core temperature of more than 38 °C), blood leukocytosis (more than 12 000 per μl) or leukopenia (less than 4000 per μl), purulent tracheal secretions, and the presence of a new or persistent radiographic infiltrate. Microbiological diagnosis is further divided by using the FOB. Non-bronchoscopic diagnosis is based on microbiological cultivation of tracheobronchial aspirate (TBAS); bronchoscopic diagnosis uses bronchoalveolar lavage or the protected brush method.
A microscopic slide from sputum and tracheobronchial aspirate is prepared, after which laboratory analysis can indicate lower respiratory tract infection through the prevalence of leukocytes and one type of microbe in the Gram stain. The material is then processed by a quantitative technique which can distinguish the presence of bacteria in the upper and lower airways. Used culture media comply with the growth of all the essential agents of pneumonia - blood agar, McConkey agar, chocolate agar, VL agar. Soils are cultivated in both aerobic atmosphere (McConkey agar) and in an atmosphere with increased CO2 tension (blood and chocolate agar) and anaerobic atmosphere (VL agar). Micro-organisms were identified using commercially produced kits: ENTEROtest 24 Lachema, STAPHYtest 24 Lachema, BBL crystal Becton Dickinson, API BioMérieux. Determination of sensitivity with regard to bacterial resistance to antibiotics was performed by the disc diffusion method, and in selected cases sensitivity was determined by the minimum inhibitory concentration (MIC) methodology recommended by the National Reference Laboratory for Antibiotics, Czech Republic.
Breakpoints for each micro-organism were determined according to CLSI (Clinical and Laboratory Standards Institute, USA) and EUCAST (European Committee on Antimicrobial Susceptibility Testing).
Results
Out of 348 adult patients hospitalized in the period of 2004-2009 at the BICU, inhalation injury was diagnosed in 127 patients (31 women/ 96 men). Table IV presents a comparison between general accepted adult BICU patients and patients with inhalation injury.
Table IV. Ratio of all adult patients admitted to the hospital intensive care unit at the Department of Burns and Reconstructive Surgery, University Hospital Brno, to patients with diagnosed inhalation injury.
The average age of patients with inhalation injury was 38.5 yr (range, 21-69 yr); average TBSA, 2-75%; average duration of hospital stay, 49.4 days (range, 4-167 days); duration of mechanical ventilation, 88.7 days. Eighteen patients (14.2%) died. In patients with inhalation injury, a total of 331 strains of non-duplicity bacteria strains were isolated from material from the lower respiratory tract. All of these bacterial strains were isolated in significant quantities for lower respiratory tract infection. Gram-negative bacterial strains totalled 227 (68.6%). The total number of bacteria, along with the number of Gram-negative bacteria in every year of the following period, is shown in Table V.
Table V. Comparison of the total number of bacteria isolated from lower respiratory tract with Gram-negative strains in the period of 2004-2009.
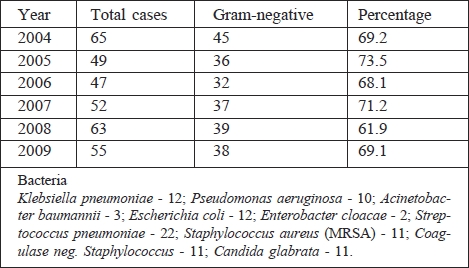
The most commonly cultivated strains of Gram-negative bacteria of lower respiratory tract infection were Klebsiella pneumoniae (78 times in all), Pseudomonas aeruginosa (49), and Acinetobacter baumanii (28). In the following period, there was a change in the spectrum of isolated Gram-negative bacteria of the lower respiratory tract. Acinetobacter baumannii, one of the dominant pathogens in 2006, has decreased since 2008. At the same time, the number of isolated Pseudomonas aeruginosa strains increased. VAT was diagnosed in 109 patients (85.83%) in the cohort. The incidence of VAT was calculated to be 98.8 per 1000 days of mechanical ventilation. VAP was diagnosed in 34 patients (26.77%), with an incidence of VAP of 30.8 cases per 1,000 days of mechanical ventilation.
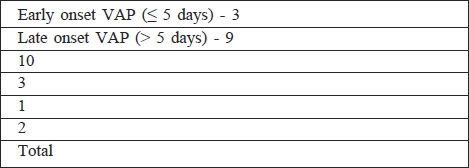
In 8 patients (23.53%) VAP was diagnosed within 5 days of initiation of mechanical ventilation, defined as early onset, while 26 patients (76.47%) had late onset. All bacteria and yeasts that caused VAP in patients in the cohort are shown in Table VI. The most common aetiological agent of VAT and VAP was Klebsiella pneumoniae (41.3%, responsible in 35.3% of patients). In two patients, VAT was caused by yeast; one patient had VAP caused by Candida glabrata. Only strains of Candida non-albicans were isolated in the lower respiratory tract of our patients.
Table VI. Various bacteria and yeasts in the aetiology of early onset VAP and late onset VAP in our patients.
Discussion
In this work we confirm that inhalation injury is not just a literary term, but a real threat to burn patients. In 348 adult patients admitted to BICU in our department, inhalation injury was diagnosed in 127 patients (36.5%). In a large retrospective study, Luo et al. diagnosed inhalation injury in 8.01% of a total of 10,608 patients admitted to hospitals in southwest China over the last two decades.16 Smith et al., in an analysis of 1447 burn patients, diagnosed inhalation injury in 284 cases (19.6%) and noted that the incidence of inhalation injury correlated with the increasing size of the burn area.17 In his recent study, Edelman confirmed increased mortality rates in patients with inhalation injury in comparison with burn patients without inhalation injury (15.88% vs 0.82%). However, a significant difference between the two groups was observed only in burn areas over 10% TBSA.18
Inhalation injury leads to the destruction of the airway lining, resulting in the loss of a number of its vital functions. Because the resulting defect opens the gateway for infection, and necrotic endothelium is an excellent growth medium for micro-organisms, it is necessary to monitor the microbiological situation regularly in every patient with this type of injury and to start a targeted therapy. Patients with inhalation injury are therefore the most vulnerable group for developing VAP.19 The incidence of VAP in our study was relatively high, corresponding to the investigated cohort of patients.
The main problem that we face is the correct diagnosis of VAP. The usual clinical data, such as fever, leukocytosis or leukopenia, purulent tracheal secretion, the presence of new or the development of already present radiographic infiltrate, have only limited informative value - particularly in patients with inhalation injury - and do not necessarily mean VAP. Pugin et al.20 combined body temperature, white blood cell count, volume, the appearance of tracheal secretions, oxygenation (PaO2/FiO2), chest Xray, and tracheal aspirate cultures into a clinical pulmonary infection score as a diagnostic tool for pneumonia in patients without the presence of inhalation injury.
Lionello et al.21 analysed the risk of death in severely burned patients in the period 1972-2000. He found that the mortality rate among patients with inhalation injury was four times as high as of than patients without inhalation injury. He established that the main cause of death was the occurrence of infectious complications in the airways as. Mlcak et al. found a 40% increase in mortality rate in cases severely burned patients with pneumonia, but if the pneumonia occurred in patients with inhalation injury the mortality rate went up to 60%.
The world's most feared respiropathogens include Pseudomonas aeruginosa and Acinetobacter baumannii. Lower respiratory tract infection by non-fermentative Gram-negative strains is burdened with a high mortality rate (22.23%) In a retrospective study at our center, we noticed the change in the spectrum of Gram-negative bacteria isolated from the lower respiratory tract in patients with inhalation injury.
Studies in 2006 showed the most dominant strains to be Acinobacter baumannii. These were replaced in 2007 by a progressively rising incidence of Pseudomonas aeruginosa. The situation in strains of Klebsiella pneumoniae was different, the frequency of capture in each year of the period being practically identical. Differences were observed only in strains producing extended-spectrum betalactamase (ESBL). While in 2006 their proportion was only 10% of the total number of isolated Klebsiellas, in 2009 it was 22.4%. None of the strains of Klebsiella pneumoniae in our patients has shown resistance to carbapenems, which in the presence of ESBL strains are the drugs of choice. Among other Gram-negative strains, where detection is not so prevalent, there are Escherichia coli and Enterobacter cloacae. In our cohort of patients, Gram-negative bacteria clearly dominated the detection of pathogens from the lower respiratory tract. Rue et al., who dealt with the incidence of nosocomial pneumonia, referred to the fact aetiologically there are prevalent strains of Gram-positive bacteria, particularly Staphylococci.24
In patients with diagnosed inhalation injury, we always use prophylactic antibiotic treatment whether they receive full pre-hospital treatment with corticosteroids or not. The choice of antibiotics is ampicillin + clavulanic acid. Administration of prophylactic antibiotics is the subject of controversy. Because there are no clear recommendations, it is up to the experience of the team of burn surgeons and microbiologists to make the choice of what, if any, prophylactic antibiotic should be given.
Conclusion
Inhalation injury is burdened with a disappointingly high morbidity and mortality rate in spite of advances in diagnostics and therapy. It is also necessary to watch for the development of infectious complications in the lower airways. Knowledge of the pathophysiology of ventilatorassociated pneumonia, along with regular microbiological screening, can lead to early detection and immediate initiation of aggressive antibiotic or antifungal therapy against the agents of ventilator-associated tracheobronchitis. pneumonia. Only these measures can minimize or avert the risk the development of ventilator-assisted pneumonia with all its consequences.
References
- 1.Sheridan R.L., Ryan C.M., Yin L.M., et al. Death in the burn unit: Sterile multiple organ failure. Burns. 1998;24:307–311. doi: 10.1016/s0305-4179(97)00062-4. [DOI] [PubMed] [Google Scholar]
- 2.Weber J.M., Sheridan R.L., Pasternack M.S., et al. Nosocomial infections in pediatric patients with burns. Am J Infect Control. 1997;25:195–201. doi: 10.1016/s0196-6553(97)90004-3. [DOI] [PubMed] [Google Scholar]
- 3.Serebrisky D., Nazarian E., Stavrinos D., et al. Inhalation injury. eMedicine.com. 2006 [Google Scholar]
- 4.Edelman D.A., White M.T., Tyburski J.G., et al. Factors affecting prognosis of inhalation injury. J Burn Care Rehabil. 2006;27:848–853. doi: 10.1097/01.BCR.0000245493.26814.CE. [DOI] [PubMed] [Google Scholar]
- 5.Lafferty K. Smoke inhalation. eMedecine.com. 2005 [Google Scholar]
- 6.Kaloudivá Y., Brychta P., Rihová H., et al. Inhalation injury. Acta Chir Plast. 2000;4:115–117. [PubMed] [Google Scholar]
- 7.Mlcak R.P., Suman O.E., Herndon D.N. Respiratory management of inhalation injury. Burns. 2007;33:2–132. doi: 10.1016/j.burns.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Carr J.A., Phillips B.D., Bowling W.M. The utility of bronchoscopy after inhalation injury complicated by pneumonia in burn patients: Results from the National Burn Repository. J Burn Care Rehabil. 2009;30:967–974. doi: 10.1097/BCR.0b013e3181bfb77b. [DOI] [PubMed] [Google Scholar]
- 9.Joseph N.M., Sistla S., Dutta T.K., et al. Ventilator-associated pneumonia: a review. Eur J Intern Med. 2010;21:360–368. doi: 10.1016/j.ejim.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Cook D.J., Walter S.D., Cook R.J., et al. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med. 1998;129:433–440. doi: 10.7326/0003-4819-129-6-199809150-00002. [DOI] [PubMed] [Google Scholar]
- 11.Rello J., Ollendorf D.A., Oster G., et al. VAP: Outcomes Scientific Advisory Group. Epidemiology and outcomes of ventilator-associated pneumonia in a large US datebase. Chest. 2002;122:2115–2121. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 12.Kollef M.H. Antimicrobial therapy of ventilator-associated pneumonia: how to select an appropriate drug regimen. Chest. 1999;115:8–11. doi: 10.1378/chest.115.1.8. [DOI] [PubMed] [Google Scholar]
- 13.Rello J., Esandi M.E., Diaz E., et al. The role of Candida sp. isolated from bronchoscopic samples in nonneutropenic patients. Chest. 1998;114:146–149. doi: 10.1378/chest.114.1.146. [DOI] [PubMed] [Google Scholar]
- 14.Klomps M., Kleinman K., Platt R. Development of an algorithm for surveillance of ventilator-associated pneumonia with electronic data and comparison of algorithm results with clinician Diagnoses. Infect Control Hosp Epidemiol. 2008;29:31–37. doi: 10.1086/524332. [DOI] [PubMed] [Google Scholar]
- 15.Pugin J., Auckenthaler R., Mili N., et al. Diagnosis of ventilatorassociated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic "blind" bronchoalveolar lavage fluid. Am Rev Respis Dis. 1991;143:1121–1129. doi: 10.1164/ajrccm/143.5_Pt_1.1121. [DOI] [PubMed] [Google Scholar]
- 16.Luo G., Peng Y., Yuan Z., et al. Inhalation injury in southwest China - the evolution of care. Burns. 2010;36:506–510. doi: 10.1016/j.burns.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Smith D.L., Cairns B.A., Ramadan F., et al. Effect of inhalation injury, burn size, and age on mortality: A study of 1447 consecutive burn patients. J Trauma. 1994;37:655–659. doi: 10.1097/00005373-199410000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Edelman D.A., White M.T., Tyburski J.G., et al. Factors affecting prognosis of inhalation injury. J Burn Care Res. 2006;27:848–853. doi: 10.1097/01.BCR.0000245493.26814.CE. [DOI] [PubMed] [Google Scholar]
- 19.Edelman D.A., Khan N., Kempf K., et al. Pneumonia after inhalation injury. J Burn Care Rehabil. 2007;28:241–246. doi: 10.1097/BCR.0B013E318031D049. [DOI] [PubMed] [Google Scholar]
- 20.Pugin J., Auckenthaler R., Mili N., et al. Diagnosis of ventilatorassociated pneumonia by bacteriologic analysis of bronchoscopic and non-bronchoscopic "blind" bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143:1121–1129. doi: 10.1164/ajrccm/143.5_Pt_1.1121. [DOI] [PubMed] [Google Scholar]
- 21.Lionelli G.T., Pickus E.J., Beckum O.K., et al. A three analysis of factors affecting burn mortality in the elderly. Burns. 2005;31:957–963. doi: 10.1016/j.burns.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Rello J., Ausina V., Ricart M., et al. Risk factors for infection by Pseudomonas aeruginosa in patients with ventilator-associated pneumonia. Intensive Care Med. 1994;20:193–198. doi: 10.1007/BF01704699. [DOI] [PubMed] [Google Scholar]
- 23.Baraibar J., Correa H., Mariscal D., et al. Risk factors for infection by Acinetobacter baumannii in intubated patients with nosocomial pneumonia. Chest. 1997;112:1050–1054. doi: 10.1378/chest.112.4.1050. [DOI] [PubMed] [Google Scholar]
- 24.Rue L.W. III, Cioffi W.G., Mason A.D., et al. The risk of pneumonia in thermally injured patients requiring ventilatory support. J Burn Care Rehabil. 1995;16:261–268. doi: 10.1097/00004630-199505000-00008. [DOI] [PubMed] [Google Scholar]


