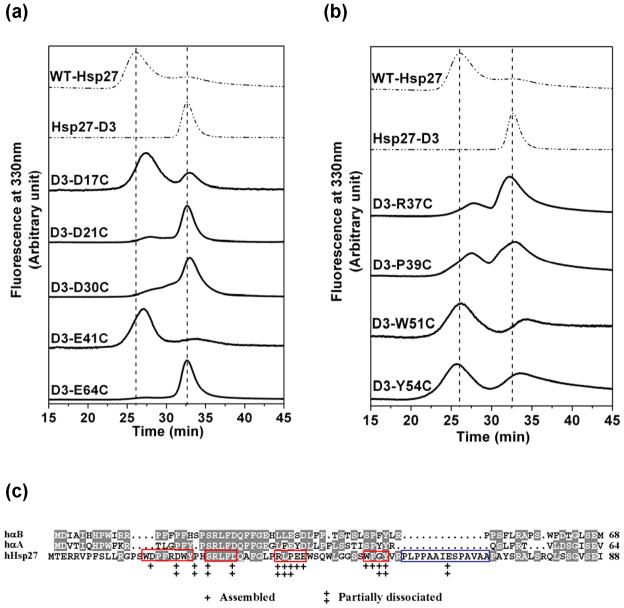
Figure 8.
SEC elution profiles of residues that influence Hsp27 dissociation equilibrium. The effect of mutation of (a) acidic residues (b) non-acidic residues on the equilibrium between large oligomers and dimers. The protein concentration was 0.05 mg/ml for (a) and 0.1 mg/ml for (b). All SEC experiments were performed on a Superose 6 column at 23°C at pH 7.2 with a flow rate of 0.5 mL/min. The dashed lines are reference chromatograms for Hsp27-WT and D3 at the same protein concentrations. (c) N-terminal sequence alignment for αB-crystallin (αB), αA-crystallin (αA), and human Hsp27. Conserved residues are shown in grey. Residues that are conserved in Hsp27 homologs are indicated by red boxes. The P1 peptide of Hsp27 is shown in blue box. Double + indicate residues that completely shift the dissociation equilibrium in favor of the native oligomer. A single plus indicates residues that partially dissociate the native oligomer.