Table 1.
Optimization studies of the reaction between o-(trimethylsilyl)phenyl triflate (1a) and N-benzylidenebenzylamine N-oxide (2a) a
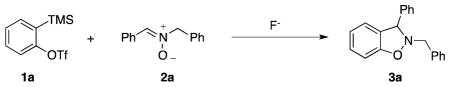 | ||||||
|---|---|---|---|---|---|---|
| entry | benzyne (equiv) | CsF (equiv) | solvent | temp. (°C) | time (h) | % yield of 3ab |
| 1 | 1.2 | 3.0 | MeCN | rt | 24 | 60 |
| 2 | 1.2 | 4.0 | MeCN | rt | 24 | 65 |
| 3 | 2.0 | 3.0 | MeCN | rt | 24 | 56 |
| 4 | 1.2 | 2.0 TBAFc | THF | rt | 24 | 45 |
| 5 | 2.0 | 4.0 TBAFc | THF | 45 | 24 | 92 |
| 6 | 1.2 | 3.0 | THF | 65 | 24 | 68 |
| 7 | 2.0 | 5.0 | THF | 65 | 14 | 93 |
| 8 | 1.6 | 4.0 | THF | 65 | 14 | 91 |
| 9 | 1.6 | 3.0 | THF | 65 | 24 | 83 |
All reactions were conducted on a 0.25 mmol scale in 5 mL of solvent for 24 h.
Yields of products isolated by column chromatography.
1M TBAF in THF solution.
