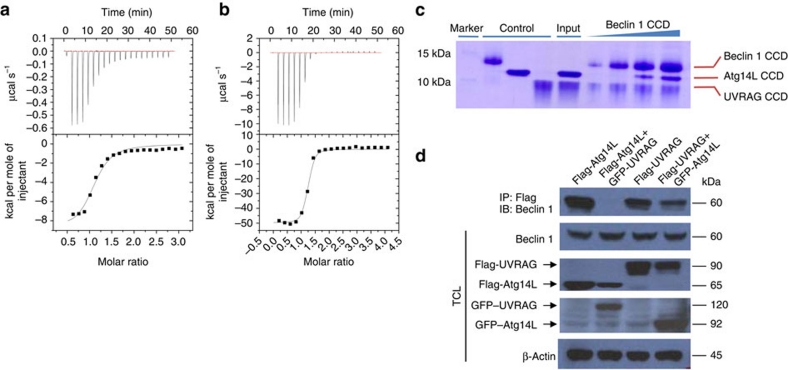
Figure 3. The Beclin 1 CC domain forms heterodimeric complex with Atg14L and UVRAG with distinct biophysical properties.
(a, b) ITC titration profile of Beclin 1 CC domain with Atg14L (a) or UVRAG (b). (c) Pull-down assay to assess the competition between Beclin 1–Atg14L and Beclin 1–UVRAG complexes. Increasing amount of His6-tagged Beclin 1 CC domain was added to a mixture of Atg14L and UVRAG proteins corresponding to their respective CC region. The Beclin 1–Atg14L/UVRAG complexes were pulled down by Ni2+– nitrilotriacetic acid (NTA) agarose beads and checked by SDS gel. (d) Co-IP experiments to assess the competition between Beclin 1–Atg14L and Beclin 1–UVRAG complexes in vivo. FLAG- or GFP-tagged Atg14L or UVRAG was transfected alone, or both were co-transfected into HEK 293T cells and their interaction with endogenous Beclin 1 was probed by co-IP using GFP antibody, followed by western blot with anti-Beclin 1 antibody.