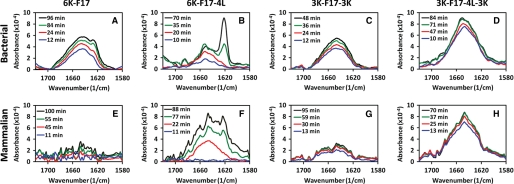
FIGURE 4.
Time-dependent ATR-FTIR spectra of synthetic CAPs in model membrane bilayers. Spectra of four CAPs (6K-F17, 6K-F17-4L, 3K-F17-3K, and 3K-F17-4L-3K) are shown in a bacterial-type (anionic) membrane lipid bilayer (3:1 POPE/DOPG) (A–D) and in a mammalian-type (zwitterionic) membrane lipid bilayer (1:1:1 DOPC/DSPC/cholesterol) (E–H). The CAP concentration was 8 μm in each spectrum. FTIR spectra for the amide I region of the CAPs reveal relative content(s) of α-helical conformation (∼1650 cm−1) (31), unordered structure (∼1640 cm−1) (22), and β-sheet-type structure (∼1625 cm−1) (30). The spectral intensity increased over time as additional amounts of CAPs penetrated the lipids, and no further changes were detected beyond the final time point in each diagram (black lines). The 6K-F17 peptide tested in E is the d-isomer; the l-enantiomer gives comparable results (data not shown).