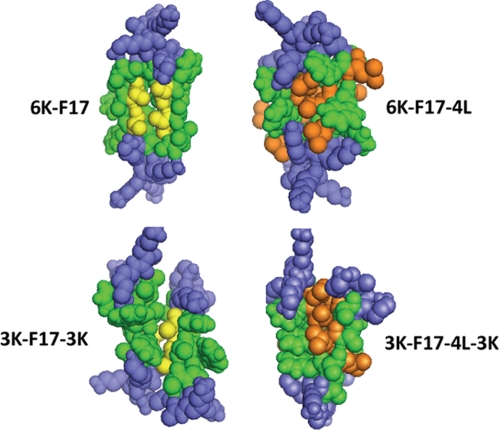
FIGURE 6.
Space-filling models of synthetic CAP dimers. Shown are representative structural models of antiparallel dimers of the four CAPs studied in this work. Structures were generated using the global conformation search program CHI. Peptides are as labeled in the diagram. Lys residues are rendered in blue; core hydrophobic residues (for both peptide molecules in the dimer) are shown in green. The packing interfaces of 6K-F17 and 3K-F17-3K are highlighted by the two (underlined) Ala residues (from each peptide molecule) in the motif “AAWAA” (rendered in gold), whereas 3K-F17-4L-3K and 6K-F17-4L have several Leu residues (from each peptide molecule) embedded in the hydrophobic binding pocket (rendered in orange).