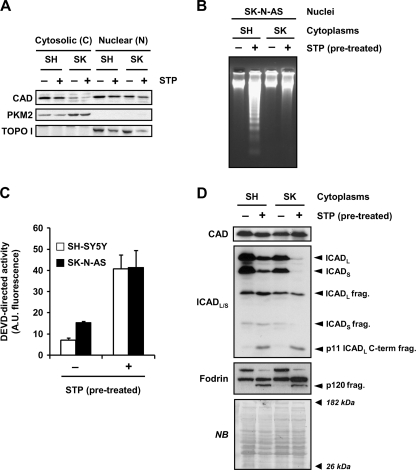
FIGURE 5.
Despite DFF40/CAD nuclear protein levels being comparable between SH-SY5Y and SK-N-AS cells, only the cytoplasm from STP-treated SH-SY5Y cells generate DNA laddering in isolated nuclei of SK-N-AS cells. A, SK-N-AS (SK) and SH-SY5Y (SH) cells treated (+) or not (−) with 1 μm STP for 6 h were subjected to a biochemical subfractionation procedure to separate the cytosolic (C) from the nuclear (N) fractions. Protein extracts were quantified, loaded onto a polyacrylamide gel, electrotransferred to PVDF membranes, and incubated with an anti-DFF40/CAD antibody (upper panel). Membranes were re-probed with an anti-PKM2 (middle panel) and an anti-topoisomerase I (TOPO I, lower panel) antibody to verify the purity of the cytosolic and nuclear fractions, respectively. B, C, and D, shown is the cell-free system assay. Cytoplasmic cell lysates were obtained from SH-SY5Y (SH) or SK-N-AS (SK) cells treated (+) or not (−) with 1 μm STP for 12 h. Isolated nuclei from untreated SK-N-AS cells were incubated for 2 h at 37 °C with 150 μg of the different cytoplasmic extracts. Then, nuclei were collected, and DNA was purified and analyzed by conventional agarose gel electrophoresis and ethidium bromide staining (B). After the cell-free system reaction, aliquots were taken, and quantitative DEVDase assay (C) or DFF40/CAD, ICAD, and α-Fodrin detection by Western blot (D) were performed. In C, DEVDase activity was carried out by incubating 25 μg of extracts with Ac-DEVD- aminofluoromethylcoumarin for 7 h in the fluorimeter. The data obtained are expressed as arbitrary units. Protein loading in D was assessed by staining the membrane with naphthol blue (NB). A.U., fluorescence arbitrary units.